Abstract
Various conditions of extraction were applied to obtain active extracts of raw poplar-type propolis. The extraction efficiency of traditional maceration was evaluated in terms of used solvent: ethanol (70 and 96% v/v), acetone (pure and 70%), propylene glycol, glycerol (50%), water and water with extraction modifiers: PEG 400 and lecithin. For obtained extracts, the total amounts of extracted phenolics and flavonoids as well as antioxidant activity were compared. For the most active extracts, the profile of volatile organic compounds with the use of GC × GC–MS and selected polyphenols content by HPLC–DAD was compared. To increase the activity of water propolis, extract ultrasound and microwave-assisted extraction were applied and obtained water extracts were compared regarding the main polyphenolic compounds content quantified by HPLC method. The recovery of 70% ethanolic extraction and the effect of the extension of extraction time were also examined by the HPTLC method. Based on conducted analyses, 70% ethanolic extract was found as the most aromatic and bioactive, followed by pure acetone and polypropylene glycol extracts. Compared to the classic maceration, water extraction assisted by microwaves and ultrasounds did not provide a higher extraction yield. In the case of 70% ethanolic extraction, the 5-day duration is recommended which allows to recovery of close to 80% of bioactive components of raw propolis.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Propolis is a bee product with recognized medicinal properties, including antioxidant, antibacterial, antifungal, antiviral, and anti-inflammatory properties. It has been proven as an effective local anesthetic, reducing spasms, healing gastric ulcers, and strengthening capillaries. It can be used internally or externally (Pujirahayu et al. 2014).
Bees collect secretions from leaf buds of trees, shrubs, and other plants, which they mix with beeswax and saliva (Bankova et al. 2000). In the hive, bees use propolis to reduce the exit opening, which provides protection against intruders, but also against wind and cold. They also use it to seal cracks in the structure of the hive (Wagh 2013). Due to the biological activity of bee glue, it protects against the development of infections inside the hive. Bees cover the bodies of dead pests too large to remove from the hive with propolis, protecting them from decay caused by bacteria (Bankova et al. 2000).
The chemical composition of propolis is very complex, which is a consequence of the way it is produced, environmental factors, such as the composition of flora and climatic conditions, are also of great importance (Hossain et al. 2022; Pobiega et al. 2023). Propolis is a sticky, gummy, resinous substance collected by honeybees with high variation in chemical compositions, generally contained over 50% (even over 70%) of resin and vegetable balsam, variable wax content (typically below 25%), essential oil and aromatics (rarely above 1%, in special cases more) (Salatino and Salatino 2021). Propolis contains more than 300 constituents and its biological activity originates from a variety of bioactive compounds, mainly polyphenols (flavonoids, phenolic acids, and their esters), terpenoids, and amino acids (Zhu et al. 2023; Woźniak et al. 2023). The composition of propolis depends on the species of honeybees and the vegetation depending on the geographic region and climatic conditions (Ristivojević et al. 2015; Hossain et al. 2022). The main plant source of European propolis is poplar, especially black poplar (Populus nigra), and therefore, it is called poplar propolis (Bankova et al. 2000; Ristivojević et al. 2015).
Propolis is a natural, renewable, and safe product with excellent potential for extending the shelf life and improving the quality of several food products (Pobiega et al. 2019a; El-Sakhawy et al. 2023). Thus, the expansion of its use as a food additive is strongly expected. Many studies have been conducted that offered different solutions, including encapsulation or selective extraction (Tosi et al. 2007; Bankova et al. 2019; El-Sakhawy et al. 2023, Tavares et al. 2022).
Because of a lot of impurities and insolubility in water, raw propolis cannot be used directly in food products and must be extracted to separate of active ingredients (Bankova et al. 2021). The propolis extracts’ chemical profile depends on the extraction solvent type, solvent ratio, and extraction procedures (Pobiega et al. 2019a). The common method of propolis processing is long-lasting maceration, and the solvent of choice for the extraction of biologically active components of propolis is 70% aqueous ethanol (Bankova et al. 2021). However, other non-ethanolic solvents have been also tested (Kubiliene et al. 2015). As the traditional maceration usually lasts about 5 days, some attempts have been made to shorten extraction times through the implementation of microwave-assisted extraction (MAE) and ultrasonic-assisted extraction (UAE) (Trusheva et al. 2007; Pobiega et al. 2019b). It has been found that the key factor is the choice of extraction solvent which influences the extract composition and consequently its biological activities (Pobiega et al. 2019b). Nevertheless, all propolis extracts will have antioxidant, antimicrobial, and anti-inflammatory activity (Šuran et al. 2021). The latest reports confirm promising data of biological action of poplar propolis extracts, including anti-inflammatory and anti-genotoxic activities, also for newly developed preparations (Acito et al. 2024). New technologies for obtaining propolis extracts are constantly being developed, e.g., using subcritical water (Shin et al. 2023), as well as new formulations allowing for a more effective delivery of bioactive ingredients to the body (Maroof et al. 2023).
The aim of the work was to search for optimal conditions for the extraction of poplar propolis, taking into account alternative solvents, techniques supporting water extraction, as well as to assess the impact of extended extraction time and multiplicity based on the chemical compositions and antioxidant activity of the obtained extracts. Multidirectional optimization, taking into account previously tested research ideas, was carried out for the first time for the same sample of high-quality raw propolis, selected in preliminary tests on the basis of biological activity.
Experimental
Reagents
DPPH (2,2-diphenyl-1-picrylhydrazyl), TPTZ (2,4,6-Tris(2-pyridyl)-s-triazine), N,O-Bis(trimethylsilyl)trifluoroacetamide, sodium carbonate, aluminum chloride, copper(II) chloride, ammonium acetate, Folin–Ciocalteu reagent were purchased from Sigma-Aldrich (Saint Louis, MO, USA), polyethylene glycol (PEG 400), neocuproine lecithin from eggs were purchased form Carl Roth GmbH (Karlsruhe, Germany). Polyphenols standards: caffeic acid, ferulic acid, benzoic acid, p-coumaric acid, pinobanksin, sakuranetin, chrysin, naringenin, galangin were obtained from Sigma-Aldrich (Saint Louis, MO, USA). Solvents: ethanol, acetone, polypropylene glycol, glycerol were obtained from Chempur (Piekary Śląskie, Poland). Solvents for chromatography (acetonitrile, ethyl acetate, chloroform) were of HPLC-grade, obtained from Honeywell Research Chemicals (Charlotte, NC, USA).
Propolis sample
The propolis sample came from an apiary located in the Podkarpackie Voivodeship (south-eastern Poland). The sample was selected based on previous analyses (data not shown) as the sample with the high content of polyphenolic compounds and high purity. Propolis was obtained from the hive using plastic grates and then recovered from them after freezing.
Extraction
Extraction using various solvents, including ethanol, acetone, propylene glycol, glycerol, water (pouring with hot water and left to macerate), PEG 400, and lecithin aqueous solutions, was carried out for a 10 g sample of crushed propolis (grinded using A10 basic grinder, IKA, Staufen, Germany), which was poured with an appropriate solvent in a volume of 100 mL. The mixtures were shaken for 30 min (400 rpm, Benchmark OrbiShaker MP) and then, left to macerate in a dark place for 5 days and then, filtered through filter paper. To test the degree of recovery, the filtration residue (5 g) was weighed and poured with 50 mL of 70% ethanol, then macerated as in the case of the first extraction. The same procedure was followed for the third extraction. To examine the influence of extraction time, the extraction time 1, 5, and 10 days were compared.
To improve water extraction efficiency, ultrasound-assisted (UAE: 15 min, temperature up to 44 °C; SONIC-10, Polsonic, Warsaw, Poland) and microwave-assisted (MAE: 4 × 15 s, power 800 W; AMM 23E80G, Amica, Wronki, Poland) extraction were applied. For comparison, the traditional maceration in a laboratory incubator (44 °C for 15 min and 24 h) was used.
Total phenolic and flavonoid content
The total phenolic content was measured using the modified procedure described by Singleton and Rossi (1965). Briefly, 0.02 mL of 100 × diluted propolis extract was mixed with 0.1 mL Folin–Ciocalteu reagent (diluted 10 ×), and next 0.08 mL of 7.5% (w/v) sodium carbonate solution was added. The reaction mixture was then incubated at room temperature for 60 min, and the absorbance was measured using EPOCH 2 microplate spectrophotometer (EPOCH 2, BioTek, Winooski, VT, USA) at 760 nm against the blank. The total phenolic content was calculated using a calibration curve (25–150 μg/mL). The results were expressed as mg of gallic acid equivalents (GAE) per 1 mL of the extract.
The total flavonoid content (TFC) was assessed using the method described by Biju et al. (2013). Briefly, 0.1 mL of the 100 × diluted propolis extract was mixed with 0.1 mL 2% AlCl3 (in methanol). The mixture was then incubated for 10 min at room temperature, and the absorbance was then measured at 415 nm with a microplate reader EPOCH 2 against methanol blank. The total content of flavonoids in the extracts was expressed in mg of quercetin equivalent (QE) per mL of extract. The results were calculated based on a calibration curve prepared 0–125 μg/mL.
Antioxidant capacity
The antioxidant capacity of extracts was assessed by three standard methods: DPPH, FRAP, and CUPRAC.
DPPH (radical scavenging activity) was measured based on the original procedure described by Blois (1958) with modifications. An aliquot of 0.02 mL of appropriate diluted propolis extract was added to 0.18 mL of 0.1 mM DPPH solution in methanol and incubated in the dark for 30 min. After this time, the absorbance was measured at 517 nm using an EPOCH 2 microplate spectrophotometer (BioTek, Winooski, VT, USA). The results were expressed as μmol Trolox equivalents per 1 mL of extract, based on the calibration curve (25–300 nmol/mL of Trolox solution in methanol).
FRAP Assay (ferric reducing antioxidant power) was performed according to Bertoncelj et al. (2007) with slight modifications. Briefly, 0.02 mL of sample was mixed with 0.18 mL FRAP reagent consisting of 2.5 mL of a 10 mM 2,4,6-tripyridyltriazine (TPTZ) solution in 40 mM HCl, 2.5 mL of 20 mM FeCl3 and 25 mL of 0.3 M acetate buffer (pH 3.6). The reaction mixture was then incubated at 37 °C, and the absorbance was measured (EPOCH 2 microplate spectrophotometer) at 593 nm. The results were calculated based on a calibration curve prepared for Trolox in the range of 25–300 nmol/mL and expressed per 1 mL of the extract.
CUPRAC assay was performed according to Apak et al. (2004) with slight modifications. Briefly, 10 μL of diluted propolis extract was mixed with 40 μL of CuCl2 (10 mM), 50 μL of neocuproine (7.5 mM), and 50 μL of ammonium acetate (1 M). The reaction mixture was then incubated at room temperature for 30 min, and the absorbance was measured with a microplate reader (EPOCH2, BioTek, Winooski, VT, USA) at 450 nm. The results were expressed as Trolox equivalents per 1 mL of extract based on a calibration curve (125–2000 nmol/mL).
GC × GC–MS analysis
The profile of volatile compounds in the most active extracts obtained with different solvents was determined by the GC × GC–MS method after the derivatization of the samples. Analyzes were performed using the Pegasus 4D device (LECO Corp.). Volatile compounds were absorbed using SPME fiber; stationary phase: divinylbenzene/carboxen/polydimethylsiloxane (DVB/CAR/PDMS); fiber length 1 cm. Phenolic compounds were determined after transformation into trimethylsilyl derivatives. 250 μL of N,O-Bis(trimethylsilyl)trifluoroacetamide was added to the weighed 5 mg solid sample, and the prepared samples were incubated at 80 °C for 1 h. After this time, 500 μL of tert-butyl methyl ether were added, and the prepared samples were subjected to GC–MS analysis. GC × GC–MS analysis conditions: Injector temperature 240 °C; first dimension furnace column BPX-5 (30 m long, 0.25 mm inner diameter, stationary phase film thickness 0.25 μm). Temperature program of the first dimension furnace: 60 °C (1 min) to 330 °C (20 min) with a temperature increase of 6 °C/minute; second dimension furnace column BPX-50 (length 2 m, inner diameter 0.1 mm, stationary phase film thickness 0.1 μm); temperature program of the second dimension furnace + 5 °C relative to the temperature of the first dimension furnace; modulator temperature program + 15 °C relative to the first dimension furnace temperature; modulation time 8 s, hot pulse time 2.4 s, cold pulse time 1.6 s (cold pulse temperature—80 °C); transfer line temperature 280 °C. Detector—mass spectrometer in full scan mode in the mass range 33–750 amu; scanning frequency 150 spectra/second; ion source temperature 200 °C. The components of extracts were identified by comparing their mass spectra with the database Wiley Registry/NIST Mass Spectral Library and Food, Flavors, Fragrances, and Related Compounds: GC–MS Library. The results were expressed as a percentage of the total peak area.
High performance liquid chromatography (HPLC) analysis
The content of selected polyphenolic components has been quantified by HPLC–DAD method using Gilson HPLC System (Gilson Inc., Middleton, WI, USA). The analytical column (Poroshell 120, EC C-18, 4.6 × 150 mm, Agilent Technologies Inc., SantaClara, CA, USA) has been applied. Extracts for analysis were filtered through 0.22 μm syringe filters and in the case of alcohol and acetone extracts diluted 5 times. A 10 µl injection was used, gradient elution mode using 0.1% formic acid in distilled water (A) and acetonitrile (B) A 10 µl injection was used, 1 mL/min flow and gradient elution mode using 0.1% formic acid in distilled water (A) and acetonitrile (B). Gradient program: 0–1.5 min 10% B, 1.5–20 min 10–100% B, 20–25 min 100% B was applied and 10% B again to equilibrate the column. The components of the extracts were identified based on a comparison of UV–Vis spectra and retention times with standards. For quantitative analysis, the standard curve method was used for the following standards: caffeic acid, p-coumaric acid, ferulic acid, benzoic acid, pinobanksin, sakuranetin, pinocembrin, and galangin. For all compounds, calibration was linear in the range 12.5–250 μg/mL (R2 > 0.997). The limit of detection (LOD) and limit of quantification (LOQ) values were calculated based on signal-to-noise (S/N) ratio: LOD as S/N = 3 and LOQ as S/N = 10. LOD values for individual compounds tested were between 0.05 and 25 μg/mL and LOQ between 1 and 50 μg/mL. The results were expressed per mL of extracts.
High performance thin layer chromatography (HPTLC) analysis
To assess the influence of maceration time and extraction times, polyphenol profiles obtained by HPTLC were analyzed. A set from Camag (Muttenz, Switzerland) was used, consisting of an applicator, an automatic developing chamber, a derivatizer, and a visualizer. The extracts were applied in a volume of 2 µL to a chromatography plate (ALUGRAM ® Xtra SIL G/UV254, Merck, Darmstadt, Germany). The chromatogram was developed using a mobile phase consisting of chloroform, ethyl acetate, and formic acid (5:4:1, v/v/v). After developing at a distance of 7 cm, the chromatogram was developed using Natural Product Reagent/PEG 400. The image was analyzed under UV light at 366 nm and processed in the VisionCats software.
Statistical analysis
All quantitative analyses were performed in triplicates, the results are given as mean and standard deviation. The correlation between the results for the obtained parameters was determined based on Pearson's coefficients. The significance of differences between the results for individual extracts was tested by Tukey's reasonable significant difference test (p = 0.05) after prior analysis of variance (ANOVA). All tests were performed using Statistica 13.3 software (StatSoft, Tulsa, OK, USA).
Results and discussion
The impact of extraction solvent during 5-day traditional maceration
In the first experiment, an attempt was made to use various solvents to obtain an active extract with high antioxidant properties and a high total content of polyphenol compounds (Table 1). As the first choice, ethanol in a mixture with water (70% v/v) was used to obtain propolis extracts (Šuran et al. 2021; Atayoglu et al. 2023). For comparison, 96% ethanol, pure acetone, and a mixture of acetone and water (70%) were applied as extraction medium. In addition to classic organic solvents, other media, suitable for the planned use of propolis extracts in the food industry were also used for extraction: propylene glycol, glycerol (50%), water, and PEG 400 and lecithin aqueous solutions.
Among the tested extracts, ethanol extracts had the highest content of phenolic compounds, including flavonoids (over half of the total polyphenol content) (Table 1). This confirms previous observations and the use of this particular solvent (in mixtures with water) to obtain propolis extracts. The comparison of ethanol at concentrations of 70 and 96% is in favor of the former solvent, except for the total phenol content; other parameters tested were significantly higher for this extract. Acetone was previously used as an extraction solvent, with different results: a similarly lower content of phenolic compounds and antioxidant status compared to the ethanol extract was observed by Bozkuş and Değer (2022), while in the research of Woźniak et al. (2020), any significant differences between acetone and ethanolic (with 70 and 96% ethanol) extracts were observed.
Moreover, due to the high volatility of acetone, ethanol is preferred as an extraction solvent. Propylene glycol, glycerol, and polyethylene glycol (PEG 400) were selected as the proposed so-called green solvents, the use of which is dictated by an attempt to eliminate classic organic solvents and obtain extracts friendly especially to the food industry. Only pure propylene glycol can be considered an effective extraction solvent based on the results. The content of phenolic compounds in this case was 4.44 mg/mL, which is a value comparable to the acetone extract and lower than for ethanol and water–acetone extracts (70%). Propylene glycol has also been previously used to extract propolis successfully; Freitas et al. (2022) obtained the content of phenolic compounds and flavonoids comparable to that for ethanol extracts, in the case of the ortho-diphenols fraction even higher, and, moreover, the efficiency of scavenging the DPPH radical, expressed by the EC50 coefficient, was even more favorable. On the other hand, the use of solvents such as propylene glycol may pose health risks: toxicity of this type of preparation has been demonstrated in studies on rats (Silici et al. 2023).
In the case of other alternative solvents, the results of the content of bioactive substances and antioxidant capacity did not differ significantly from those for the water extract, which indicates poor efficiency and limited possibilities of practical application. The use of anhydrous and aqueous polyethylene glycol solutions (20%) for the extraction of poplar propolis from Lithuania has been previously described. The content of total polyphenols as well as the determined individual phenolic acids in such extracts was significantly lower than in the classic 70% ethanol extract, although the antioxidant properties examined against in vitro cell culture were similar for all tested extracts (Kubiliene et al. 2018). More favorable parameters were also obtained using anhydrous PEG 400. Despite obtaining a lower extraction efficiency, the polyphenol content and antioxidant activity of such extracts were comparable to the ethanolic extract; moreover, better activity against E. coli was observed. This solvent is therefore postulated as promising, even for demanding biomedical applications (Šuran et al. 2021). The authors explain the better solubilization of polyphenolic compounds by the formation of a network of hydrogen bonds between the solvent and numerous hydroxyl groups of flavonoids, such as kaempferol (Šuran et al. 2021).
A relatively new idea is the use of lecithin as a water extraction modifier. Due to the emulsifying properties of lecithins, it can be expected that their solutions can be effective extraction agents. There is a known patent in which 0.1–3.5% concentration of soy lecithin was used to extract propolis, obtaining an extract containing the basic bioactive components of propolis: p-coumaric acid, ferulic acid, caffeic acid, and CAPE (Radić et al. 2020). Lecithin was also used to extract Tetragonula sp. propolis, but this solvent turned out to be less effective than olive oil, virgin coconut oil or propylene glycol (Christina et al. 2018). Attempts have also been made to use lecithin as a natural surfactant to isolate polyphenols from other natural raw materials, e.g., peach waste using the cloud point extraction method (Giovanoudis et al. 2023). However, the results obtained in our work are unsatisfactory and indicate the need to optimize this type of green extraction.
The results of the antioxidant potential of tested propolis extracts obtained by three methods significantly correlated with the content of total polyphenols and flavonoids (r > 0.9), which proves the dominant role of these compounds in shaping this bioactivity (Table 2). All correlation coefficients were significant at p = 0.05.
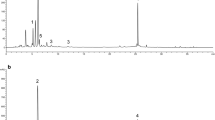
Selected extracts with high antioxidant activity were analyzed using HPLC–DAD technique. The results of the quantitative analysis of selected identified phenolic acids and flavonoids are presented in Fig. 1.
In the extracts analyzed by HPLC–DAD, mainly phenolic acids (caffeic, p-coumaric, ferulic, benzoic and their derivatives) and flavonoids (pinobanksin, sakuranetin, pinocembrin, galangin, and other compounds from this group) were detected. Those identified with high certainty by comparing spectra and retention times with available standards were subjected to quantitative analysis. The obtained results indicate that the acetone extract has the highest content of the determined phenolic acids, and the ethanol extract has a slightly lower content (70%). The differences are particularly visible in the case of the dominant p-coumaric acid, which in these extracts was determined to be 3.62 and 3.43 mg/mL, respectively. In the case of extraction with propylene glycol, the content of p-coumaric acid was lower, at the level of 2.49 mg/mL of the extract. In the case of caffeic, ferulic, and benzoic acids, their content in 70% ethanol and acetone extracts did not differ significantly. Phenolic acids were almost the only compounds present in the water extract, apart from flavonoids only pinobanksin was detected in small amounts. However, in terms of the content of these compounds, this extract was richer than that prepared with a 50% glycerol solution. The determined compounds from the flavonoid group were most effectively extracted with ethanol (regardless of concentration), acetone, and propylene glycol, for which their contents were comparable, with the exception of sakuranetin, which was significantly highest in the acetone extract (1.95 mg/mL). Unfortunately, CAPE could not be determined in the analyzed extracts, probably due to co-elution with other compounds.
The data obtained regarding the polyphenol composition of the extracts include compounds commonly determined in propolis from this region of Europe. However, other authors identified additionally in Polish propolis cinnamic, gallic, hydroxybenzoic, gentisic, vanillic, and chlorogenic acids and their numerous derivatives (Socha et al. 2015; Woźniak et al. 2019; Pobiega et al. 2023). The group of flavonoids also includes, among others: apigenin, chrysin, kaempferol, myricetin, naringenin, quercetin, pinostrobin, hesperetin (Socha et al. 2015; Woźniak et al. 2019; Pobiega et al. 2023). A previous comparison of the extraction of Polish propolis using 70 and 96% ethanol shows a similar tendency as in our study, significantly fewer phenolic acids and flavonoids were determined in the extract with 96% ethanol (Woźniak et al. 2019).
The same extracts were examined by GC × GC–MS in terms of volatile aroma components. Semi-quantitative profiles of these samples are summarized in Table 3.
An example of chromatogram for ethanolic (70%) extract in the form of a two-dimensional map is shown in Fig. 2. The analyses showed that the ethanol extract (96%) was richest in volatile compounds; slightly less intense signals were present in the chromatograms of extracts prepared using 70% ethanol. Among the identified compounds, derivatives of benzoic and cinnamic acid predominated, and there were also flavonoids (pinocembrin, pinostrobin, tectochrysin). A significant percentage of the profiles in the case of acetone and ethanol extracts (96%) was aliphatic components of waxes (octacosanol and pentacosane). The presence of identified compounds in propolis from Poland and neighboring countries was previously confirmed, stating that a large number of them originate from poplar, aspen, or birch (Isidorov et al. 2014; Popova et al. 2017; Moskwa et al. 2020). No volatile odor compounds were detected in the water extract, and only 2 compounds (2,3-dihydrobenzofurane and 2-methoxy-4-vinylphenol) were detected in the glycerol extract (50%). The polypropylene glycol extract was slightly richer, and contained numerous derivatives of benzoic acid and a characteristic odor compound: p-vinylguaiacol. This compound was detected as one of the important ones in Turkish propolis (Donmez et al. 2020; Arslan et al. 2021). When propylene glycol and glycerol were used as extraction solvents, these compounds dominated among the identified ingredients.
To our knowledge, chromatographic (HPLC–DAD and GC × GC–MS) comparisons of profiles for crude propolis extracts prepared using a wide range of solvents were described for the first time.
The effect of extraction technique on aqueous extraction of propolis
As water extraction of propolis is not effective and meanwhile water is still considered the best solvent for applications in food technology and medicine, the influence of supporting water extraction with physical factors on improving the recovery of bioactive substances from propolis was checked. The test results are summarized in Table 4.
The best effect was obtained using microwave support, the total content of phenols and flavonoids as well as the antioxidant capacity of the extract prepared in this way were approximately 5 times higher than that obtained with ultrasound-assisted extraction. The use of water maceration at a temperature of 44 °C (the same as obtained during ultrasound support) did not bring a positive effect. Only extending the water maceration time to 24 h increases the effectiveness of antioxidant extraction. Interestingly, extraction at 44 °C for 24 h was even 3 times more effective than 5-day extraction in water at room temperature. The best efficiency of the microwave-assisted extraction was confirmed by HPLC–DAD analysis (Fig. 3). Unlike extraction with organic solvents, mainly phenolic acids and only pinobanksin among the flavonoids pass into the water. The same was previously observed for aqueous extraction (Fig. 1). Water extraction of propolis was previously investigated by Nagai et al. (2003), who determined that, despite its lower bioactive substance content, such extract may have potential in pharmaceutical applications. However, it has previously been shown that propolis extracts prepared with pure water as well as 10 and 20% ethanol do not have antimicrobial properties (Park and Ikegaki 1998).
The effectiveness of 70% ethanolic extraction in terms of duration time and recovery
Another factor that may have a significant impact on the recovery of bioactive compounds from propolis may be the extraction time. Maceration efficiency within one, five, and ten days was compared. It can be concluded that 1-day maceration is less effective than 5-day maceration, but extending the time to 10 days does not bring a significant increase in the polyphenol content and antioxidant capacity of the obtained propolis extract (Table 5). Interesting results were obtained by examining the effect of re-extraction of the residue after the first extraction. It turned out that the polyphenol content in this second extraction was as much as 23% of what was extracted in the first attempt. This is a large amount, so it can be concluded that repeating the extraction makes sense to increase the recovery of bioactive substances. In the case of one more extraction, another almost 6% of the phenol content was recovered.
The same was observed by comparison of polyphenolic profiles using HPTLC method (Fig. 4). The profiles obtained regardless of the extraction duration (tracks 1, 2, and 3), are similar in terms of qualitative composition, there are 8 main bands corresponding to individual flavonoids and phenolic acids of propolis. However, in the case of 1-day maceration, the intensity of the bands is visibly lower, which correlates with the quantitative data presented above. In the case of repeated extraction (tracks 2, 4, and 5), the full composition of the bands is also visible, while in the third extraction only some, weakly intense bands are visible. The performed analysis confirms the usefulness of the HPTLC technique for the preliminary assessment of extraction efficiency based on the intensity of the separated bands. It was previously used to compare propolis extracts of various origins and to assess bioactivity by bioautography (Bertrams et al. 2013; Milojković-Opsenica et al. 2016; Ristivojević et al. 2015; Guzelmeric et al. 2018; Miłek et al. 2022).
Conclusions
Among the tested extraction systems, the highest extraction efficiency was achieved for 70% ethanol, which supports the current theory of propolis extraction. Similar extraction efficiency was achieved for pure acetone and propylene glycol, but these solvents are problematic in applications in the food industry.
The used water extraction modifiers (PEG 400 and lecithin) did not increase the extraction efficiency, nor did the use of supporting physical factors, among which microwave-assisted extraction seems to be worth optimizing.
Extending the ethanol maceration time to 10 days did not increase the extraction efficiency, but it was shown that one-stage extraction allows washing out about 80% of the bioactive ingredients, therefore re-extraction of the residue is necessary and economically justified.
References
Acito M, Varfaj I, Brighenti V, Cengiz EC, Rondini T, Fatigoni C, Russo C, Pietrella D, Pellati F, Bartolini D, Sardella R, Moretti M, Villarini M (2024) A novel black poplar propolis extract with promising health-promoting properties: focus on its chemical composition, antioxidant, anti-inflammatory, and anti-genotoxic activities. Food Funct. https://doi.org/10.1039/d3fo05059a
Apak R, Güclü K, Özyürek M, Karademir SE (2004) Novel total antioxidant capacity index for dietary polyphenols and vitamins C and E, using their cupric ion reducing capability in the presence of neocuproine: CUPRAC method. J Food Agric Chem 52:7970–7981
Arslan M, Sevgiler Y, Güven C, Murathan ZT, Erbil N, Ylldlrlm D, Büyükleyla M, Karadaş Ş, Çelik R, Rencüzoǧullarl E (2021) Chemical and biological characteristics of propolis from Apis mellifera caucasica from the Ardahan and Erzurum provinces of Turkey: a comparative study. Arh Hig Rada Toksikol 72:53–69. https://doi.org/10.2478/aiht-2021-72-3492
Atayoglu AT, Sözeri Atik D, Bölük E, Gürbüz B, Ceylan FD, Çapanoğlu E, Atayolu R, Paradkar A, Fearnley J, Palabiyik I (2023) Evaluating bioactivity and bioaccessibility properties of the propolis extract prepared with L-lactic acid: an alternative solvent to ethanol for propolis extraction. Food Biosci 53:102756. https://doi.org/10.1016/j.fbio.2023.102756
Bankova V, de Casto S, Marcucci M (2000) Propolis: recent advances in chemistry and plant origin. Apidologie 31:3–15. https://doi.org/10.1051/apido:2000102
Bankova V, Bertelli D, Borba R, Conti BJ, da Silva Cunha IB, Danert C, Eberlin MN, Falcão SI, Isla MI, Moreno MIN, Papotti G, Popova M, Santiago KB, Salas A, Sawaya ACHF, Schwab NV, Sforcin JM, Simone-Finstrom M, Spivak M, Trusheva B, Vilas-Boas M, Wilson M, Zampini C (2019) Standard methods for Apis mellifera propolis research. J Apic Res 58:1–49. https://doi.org/10.1080/00218839.2016.1222661
Bankova V, Trusheva B, Popova M (2021) Propolis extraction methods: a review. J Apic Res 60:734–743. https://doi.org/10.1080/00218839.2021.1901426
Bertoncelj J, Doberšek U, Jamnik M, Golob T (2007) Evaluation of the phenolic content, antioxidant activity and colour of Slovenian honey. Food Chem 105:822–828. https://doi.org/10.1016/J.FOODCHEM.2007.01.060
Bertrams J, Müller MB, Kunz N, Kammerer DR, Stintzing FC (2013) Phenolic compounds as marker compounds for botanical origin determination of German propolis samples based on TLC and TLC-MS. J Appl Bot Food Qual 86:143–153. https://doi.org/10.5073/JABFQ.2013.086.020
Biju J, Reddy V, Sulaiman CT (2013) Total phenolics and flavonoids in selected Justicia species. J Pharmacogn Phytochem 2:51–52
Blois MS (1958) Antioxidant determinations by the use of a stable free radical. Nature 181:1199–1200. https://doi.org/10.1038/1811199a0
Bozkuş TN, Değer O (2022) Comparison of total phenolic contents and antioxidant activities of propolis in different solvents. Food Health 8:111–117. https://doi.org/10.3153/fh22011
Christina D, Hermansyah H, Wijanarko A, Rohmatin E, Sahlan M, Pratami DK, Mun’Im A (2018) Selection of propolis Tetragonula sp. extract solvent with flavonoids and polyphenols concentration and antioxidant activity parameters. AIP Conf Proc. https://doi.org/10.1063/1.5023967
Donmez M, Karadeniz S, Yoldas T, Aydin G, Karagul P, Aksu O, Rasgele PG (2020) Comparison of chemical contents of extracts in different solvents of propolis samples produced in Duzce Province. Int J Tradit Complement Med Res 1:137–146
El-Sakhawy M, Salama A, Mohamed SAA (2023) Propolis applications in food industries and packaging. Biomass Convers Biorefinery. https://doi.org/10.1007/s13399-023-04044-9
Freitas AS, Cunha A, Parpot P, Cardoso SM, Oliveira R, Almeida-Aguiar C (2022) Propolis efficacy: the quest for eco-friendly solvents. Molecules 27(21):7531. https://doi.org/10.3390/molecules27217531
Giovanoudis I, Athanasiadis V, Chatzimitakos T, Kalompatsios D, Bozinou E, Gortzi O, Nanos GD, Lalas SI (2023) Isolation of polyphenols from two waste streams of clingstone peach canneries utilizing the cloud point extraction method. Biomass (switz) 3:291–305. https://doi.org/10.3390/biomass3030018
Guzelmeric E, Ristivojević P, Trifković J, Dastan T, Yilmaz O, Cengiz O, Yesilada E (2018) Authentication of Turkish propolis through HPTLC fingerprints combined with multivariate analysis and palynological data and their comparative antioxidant activity. LWT 87:23–32. https://doi.org/10.1016/j.lwt.2017.08.060
Hossain R, Quispe C, Khan RA, Saikat ASM, Ray P, Ongalbek D, Yeskaliyeva B, Jain D, Smeriglio A, Trombetta D, Kiani R, Kobarfard F, Mojgani N, Saffarian P, Ayatollahi SA, Sarkar C, Islam MT, Keriman D, Uçar A, Martorell M, Sureda A, Pintus G, Butnariu M, Sharifi-Rad J, Cho WC (2022) Propolis: an update on its chemistry and pharmacological applications. Chin Med (u k). https://doi.org/10.1186/s13020-022-00651-2
Isidorov VA, Szczepaniak L, Bakier S (2014) Rapid GC/MS determination of botanical precursors of eurasian propolis. Food Chem 142:101–106. https://doi.org/10.1016/j.foodchem.2013.07.032
Kubiliene L, Laugaliene V, Pavilonis A, Maruska A, Majiene D, Barcauskaite K, Kubilius R, Kasparaviciene G, Savickas A (2015) Alternative preparation of propolis extracts: comparison of their composition and biological activities. BMC Complement Altern Med 15:1–7. https://doi.org/10.1186/s12906-015-0677-5
Kubiliene L, Jekabsone A, Zilius M, Trumbeckaite S, Simanaviciute D, Gerbutaviciene R, Majiene D (2018) Comparison of aqueous, polyethylene glycol-aqueous and ethanolic propolis extracts: antioxidant and mitochondria modulating properties. BMC Complement Altern Med 18:1–10. https://doi.org/10.1186/s12906-018-2234-5
Maroof K, Lee RFS, Siow LF, Goh BH, Chen KF, Gan SH (2023) A new stable and bioactive formulation of Geniotrigona thoracia propolis microemulsion for oral delivery. Food Chem Adv 3:100514. https://doi.org/10.1016/j.focha.2023.100514
Miłek M, Ciszkowicz E, Tomczyk M, Sidor E, Zaguła G, Lecka-Szlachta K, Pasternakiewicz A, Dżugan M (2022) The study of chemical profile and antioxidant properties of poplar-type polish propolis considering local flora diversity in relation to antibacterial and anticancer activities in human breast cancer cells. Molecules 27:725. https://doi.org/10.3390/molecules27030725
Milojković Opsenica D, Ristivojević P, Trifković J, Vovk I, Lušić D, Tešić Ž (2016) TLC fingerprinting and pattern recognition methods in the assessment of authenticity of poplar-type propolis. J Chromatogr Sci 54:1077–1083. https://doi.org/10.1093/chromsci/bmw024
Moskwa J, Naliwajko SK, Markiewicz-Żukowska R, Gromkowska-Kępka KJ, Nowakowski P, Strawa JW, Borawska MH, Tomczyk M, Socha K (2020) Chemical composition of Polish propolis and its antiproliferative effect in combination with Bacopa monnieri on glioblastoma cell lines. Sci Rep 10:1–16. https://doi.org/10.1038/s41598-020-78014-w
Nagai T, Inoue R, Inoue H, Suzuki N (2003) Preparation and antioxidant properties of water extract of propolis. Food Chem 80(1):29–33. https://doi.org/10.1016/S0308-8146(02)00231-5
Park YK, Ikegaki M (1998) Preparation of water and ethanolic extracts of propolis and evaluation of the preparations. Biosci Biotechnol Biochem 62:2230–2232. https://doi.org/10.1271/bbb.62.2230
Pobiega K, Kraśniewska K, Gniewosz M (2019a) Application of propolis in antimicrobial and antioxidative protection of food quality—a review. Trends Food Sci Technol 83:53–62. https://doi.org/10.1016/j.tifs.2018.11.007
Pobiega K, Kraśniewska K, Derewiaka D, Gniewosz M (2019b) Comparison of the antimicrobial activity of propolis extracts obtained by means of various extraction methods. J Food Sci Technol 56:5386–5395. https://doi.org/10.1007/s13197-019-04009-9
Pobiega K, Kot AM, Przybył JL, Synowiec A, Gniewosz M (2023) Comparison of the chemical composition and antioxidant properties of propolis from urban apiaries. Molecules 28:6744. https://doi.org/10.3390/molecules28186744
Popova M, Giannopoulou E, Skalicka-Wózniak K, Graikou K, Widelski J, Bankova V, Kalofonos H, Sivolapenko G, Gaweł-Bȩben K, Antosiewicz B, Chinou I (2017) Characterization and biological evaluation of propolis from Poland. Molecules 22:1159. https://doi.org/10.3390/molecules22071159
Pujirahayu N, Ritonga H, Uslinawaty Z (2014) Properties and flavonoids content in propolis of some extraction method of raw propolis. Int J Pharm Pharm Sci 6:338–340
Radić S, Radić B, Šuran J (2020) Liquid propolis extract, its preparation and use thereof. WO patent 2020169425A1, Aug 27, 2020
Ristivojević P, Trifković J, Andrić F, Milojković-Opsenica D (2015) Poplar-type propolis: chemical composition, botanical origin and biological activity. Nat Prod Commun 10:1869–1876. https://doi.org/10.1177/1934578x1501001117
Salatino A, Salatino MLF (2021) Scientific note: often quoted, but not factual data about propolis composition. Apidologie 52:312–314. https://doi.org/10.1007/s13592-020-00821-x
Shin SB, Lee JK, Ko MJ (2023) Enhanced extraction of bioactive compounds from propolis (Apis mellifera L.) using subcritical water. Sci Rep 13(1):15038. https://doi.org/10.1038/s41598-023-42418-1
Silici S, Kaynar L, Okan A, Doğanyiğit Z (2023) Protective role of olive oil extract of propolis on short and long-term administration of tamoxifen in rats. Environ Toxicol 39:2102–2122. https://doi.org/10.1002/tox.24091
Singleton VL, Rossi JA (1965) Colorimetry of total phenolics with phosphomolybdic-phosphotungstic acid reagents. Am J Enol Vitic 16:144–158
Socha R, Gałkowska D, Bugaj M, Juszczak L (2015) Phenolic composition and antioxidant activity of propolis from various regions of Poland. Nat Prod Res 29:416–422. https://doi.org/10.1080/14786419.2014.949705
Šuran J, Cepanec I, Mašek T, Radić B, Tlak Gajger I, Vlainić J (2021) Propolis extract and its bioactive compounds—from traditional to modern extraction technologies. Molecules 26:2930. https://doi.org/10.3390/molecules26102930
Tavares L, Smaoui S, Lima PS, de Oliveira MM, Santos L (2022) Propolis: encapsulation and application in the food and pharmaceutical industries. Trends Food Sci Technol 127:169–180. https://doi.org/10.1016/j.tifs.2022.06.003
Tosi EA, Ré E, Ortega ME, Cazzoli AF (2007) Food preservative based on propolis: bacteriostatic activity of propolis polyphenols and flavonoids upon Escherichia coli. Food Chem 104:1025–1029. https://doi.org/10.1016/j.foodchem.2007.01.011
Trusheva B, Trunkova D, Bankova V (2007) Different extraction methods of biologically active components from propolis; a preliminary study. Chem Cent J 1:1–4. https://doi.org/10.1186/1752-153X-1-13
Wagh VD (2013) Propolis: A wonder bees product and its pharmacological potentials. Adv Pharmacol Sci 2013:308249. https://doi.org/10.1155/2013/308249
Woźniak M, Mrówczyńska L, Waśkiewicz A, Rogoziński T, Ratajczak I (2019) Phenolic profile and antioxidant activity of propolis extracts from Poland. Nat Prod Commun 14:1–7. https://doi.org/10.1177/1934578X19849777
Woźniak M, Mrówczyńska L, Kwaśniewska-Sip P, Waśkiewicz A, Nowak P, Ratajczak I (2020) Effect of the solvent on propolis phenolic profile and its antifungal, antioxidant, and in vitro cytoprotective activity in human erythrocytes under oxidative stress. Molecules 25:4266. https://doi.org/10.3390/molecules25184266
Woźniak M, Sip A, Mrówczyńska L, Broniarczyk J, Waśkiewicz A, Ratajczak I (2023) Biological activity and chemical composition of propolis from various regions of Poland. Molecules 28:141. https://doi.org/10.3390/molecules28010141
Zhu L, Zhang J, Yang H, Li G, Li H, Deng Z, Zhang B (2023) Propolis polyphenols: a review on the composition and anti-obesity mechanism of different types of propolis polyphenols. Front Nutr 10:1–14. https://doi.org/10.3389/fnut.2023.1066789
Acknowledgements
The research was financially supported by Podkarpackie Innovation Center Project PCI N3_125 “A method of producing organoleptically neutral bio-preservative based on propolis” and Polish Ministry of Science and Higher Education research project within the University of Rzeszów PB/ZCHTZ/2023.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
On behalf of all authors, the corresponding author states that there is no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Miłek, M., Bonikowski, R. & Dżugan, M. The effect of extraction conditions on the chemical profile of obtained raw poplar propolis extract. Chem. Pap. (2024). https://doi.org/10.1007/s11696-024-03567-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11696-024-03567-3