Abstract
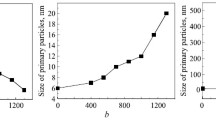
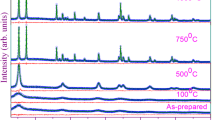
Structure-stability correlations in terms of microstructure related to tin oxidation were determined using an in situ high-temperature X-ray diffraction. Tin oxidation takes place over a broad temperature range between 300 and 900 °C, which resulted in SnO at 300 °C and SnO2 that is thermodynamically more stable phase above 500 °C. The initial conversion from tin to SnO ultimately resulted in a matrix expansion by 36%, which is mainly due to unit cell volume contraction, resulting in a temporary decrease in tin lattice strain. The tin unit cell volume is rapidly increased during the initial heating, decreased slightly during the formation of SnO, and changed marginally during the oxidation to SnO2.The tin lattice strain significantly increased from 0.84 × 10–4 to 4.76 × 10–4 and crystallite size decreased by 56% during the entire period of oxidation, indicating the ongoing lattice destabilization under thermal excitation. At high temperatures, SnO2 is the most stable oxide form of tin, which is stabilized under the low lattice strain of 9.30 × 10–5 and a large crystallite size of 39 nm that is mainly achieved via compact unit cell arrangement in space. The SnO has a strong orientation preference in the (112) direction, and as temperature increased, the orientation preference along this direction decreased. The stable SnO2 exhibits a strong crystallite orientation preference along the (111) direction, which intensified with temperature, resulting in a preferred crystallite growth along this direction.
Graphical abstract





Similar content being viewed by others
References
Abban S, Ikumapayi OM, Ogedengbe TS, Afolalu SA, Ananaba C, Ogundipe AT (2023) Development of aluminium-tin alloy for high strength application. Mater Today Proc
Al-Had NM, Kamari HM, Baqer AA, Shaari AH, Saion E (2018) Thermal calcination-based production of SnO2 nanopowder: an analysis of SnO2 nanoparticle characteristics and antibacterial activities. Nanomaterials 8:250. https://doi.org/10.3390/nano8040250
Aziz M, Abbas SS, Baharom WRW, Mahmud WZW (2012) Structure of SnO2 nanoparticles by sol–gel method. Mater Lett 74:62–64
Batzill M, Diebold U (2005) The surface and materials science of tin oxide. Prog Surf Sci 79(2):47–154
Boggs WE, Trozzo PS, Pellissier GE (1961) The oxidation of Tin: II. The morphology and mode of growth of oxide films on Pure Tin. J Electrochem Soc 108(1):13
Cahen S, David N, Fiorani JM, Maitre A, Vilasi M (2003) Thermodynamic modelling of the O–Sn system. Thermochim Acta 403(2):275–285
Chen JS, Lou XW (2013) SnO2-based nanomaterials: synthesis and application in lithium-ion batteries. Small 9(11):1877–1893
Cho S, Yu J, Kang SK, Shih D-Y (2005) Oxidation study of pure tin and its alloys via electrochemical reduction analysis. J Electron Mater 34(5):635–642
Choi SH, Kang YC (2014) Kilogram-scale production of SnO2 yolk-shell powders by a spray-drying process using dextrin as carbon source and drying additive. Chem Eur J 20(19):5835–5839
Cojocaru B, Avram D, Kessler V, Parvulescu V, Seisenbaeva G, Tiseanu C (2017) Nanoscale insights into doping behavior, particle size and surface effects in trivalent metal doped SnO2. Sci Rep 7(1):9598
Jia X, Lin Z, Yang TC, Puthen-Veettil B, Wu L, Conibeer G, Perez-Wurfl I (2018) Post-sputtering heat treatments of molybdenum on silicon wafer. Appl Sci 8:1692. https://doi.org/10.3390/app8091692
Jiang L, Sun G, Zhou Z, Sun S, Wang Q, Yan S, Li H, Tian J, Guo J, Zhou B, Xin Q (2005) Size-controllable synthesis of monodispersed SnO2 nanoparticles and application in electrocatalysts. J Phys Chem B 109(18):8774–8778
Kameshima Y, Tamura Y, Nakajima A, Okada K (2020) Electroplating of zinc and tin alloys with nickel and cobalt from ammonium oxalate electrolytes. Russ Chem Bull 69(7):1272–1278
Karunadasa KSP (2019) Dehydration of calcium chloride as examined by high-temperature X-ray powder diffraction. Int Multidiscip Res J 4:37–43
Karunadasa KSP, Manoratne CH (2022) Microstructural view of anatase to rutile phase transformation examined by in-situ high-temperature X-ray powder diffraction. J Solid State Chem 314:123377
Karunadasa KSP, Manoratne CH, Pitawala HMTGA, Rajapakse RMG (2019) Thermal decomposition of calcium carbonate (calcite polymorph) as examined by in-situ high-temperature X-ray powder diffraction. J Phys Chem Sol 134:21–28
Karunadasa KSP, Rajapakse RMG, Pitawala HMTGA, Manoratne CH (2023) Microstructural insight into the thermal decomposition of MgCl2·6H2O examined by in-situ high-temperature X-ray powder diffraction. J Solid State Chem 322:123965
Kasper E, Werner J et al (2012) Growth of silicon based germanium tin alloys. Thin Solid Films 520(8):3195–3200
Kilby KT, Jiao S, Fray DJ (2010) Current efficiency studies for graphite and SnO2-based anodes for the electro-deoxidation of metal oxides. Electrochim Acta 55(23):7126–7133
Korotcenkov G, Cho BK (2012) Ozone measuring: What can limit application of SnO2-based conductometric gas sensors? Sens Actuators B Chem 161(1):28–44
Kumar GR, Sathishkumar M, Vignesh M, Manikandan M, Rajyalakshmi G, Ramanujam R, Arivazhagan N (2023) Metal additive manufacturing of commercial aerospace components–A comprehensive review. Proc Inst Mech Eng Part E J Process Mech Eng 237(2):441–454
Lee S, Ocon JD, Son YI, Lee J (2015) Alkaline CO2 electrolysis toward selective and continuous HCOO–production over SnO2 nanocatalysts. J Phys Chem C 119(9):4884–4890
Leitner J, Sedmidubský D (2019) Thermodynamic modeling of oxidation of tin nanoparticles. J Phase Equilib Diffus 40(1):10–20
Li F, Song J, Yang H, Gan S, Zhang Q, Han D, Ivaska A, Niu L (2009) One-step synthesis of graphene/SnO2 nanocomposites and its application in electrochemical supercapacitors. Nanotechnology 20(45):455602
Liu Y, Zheng C, Wang W, Zhan Y, Wang G (2001) Production of SnO2 nanorods by redox reaction. J Cryst Growth 233(1):8–12
Matussin S, Harunsani MH, Tan AL, Khan MM (2020) Plant-extract-mediated SnO2 nanoparticles: synthesis and applications. ACS Sustain Chem Eng 8(8):3040–3054
Miller SA, Gorai P, Aydemir U, Mason TO, Stevanović V, Toberer ES, Snyder GJ (2017) SnO as a potential oxide thermoelectric candidate. J Mater Chem C 5(34):8854–8861
Molloy KC (2009) Tin chemistry: fundamentals, frontiers, and applications. J Am Chem Soc 131(14):5361–5362
Mote VD, Purushotham Y, Dole BN (2012) Williamson-Hall analysis in estimation of lattice strain in nanometer-sized ZnO particles. J Theor Appl Phys 6(1):6
Ning J, Jiang T, Men K, Dai Q, Li D, Wei Y, Liu B, Chen G, Zou B, Zou G (2009) Syntheses, characterizations, and applications in lithium ion batteries of hierarchical SnO nanocrystals. J Phys Chem C 113(32):14140–14144
Ostrakhovitch EA (2022) Chapter 33 - Tin. In: Nordberg GF, Costa M (eds) Handbook on the toxicology of metals, 4th edn. Academic Press, pp 807–856
Poulier C, Smith DS, Absi J (2007) Thermal conductivity of pressed powder compacts: tin oxide and alumina. J Eur Ceram 27(2):475–478
Ren Y, Li S, Lv Z, Fan Y, He J, Song J (2023) Electrolysis synthesis of carbides and carbon dioxide capture in molten salts. Small 19(23):2207863
Saji KJ, Venkata Subbaiah YP, Tian K, Tiwari A (2016) P-type SnO thin films and SnO/ZnO heterostructures for all-oxide electronic and optoelectronic device applications. Thin Solid Films 605:193–201
Sharma V, Vyas R, Bazylewski P, Chang GS, Asokan K, Sachdev K (2016) Probing the highly transparent and conducting SnOx/Au/SnOx structure for futuristic TCO applications. RSC Adv 6(35):29135–29141
Song P, Wen D (2009) Experimental Investigation of the Oxidation of Tin Nanoparticles. J Phys Chem C 113(31):13470–13476
Wang H, Rogach AL (2014) Hierarchical SnO2 nanostructures: recent advances in design, synthesis, and applications. Chem Mater 26(1):123–133
Xiong L, Guo Y, Wen J, Liu H, Yang G, Qin P, Fang G (2018) Review on the application of SnO2 in perovskite solar cells. Adv Funct Mater 28(35):1802757
Yang F, Li JCM (2007) Deformation behavior of tin and some tin alloys. J Mater Sci Mater Electron 18(1):191–210
Yin H, Mao X, Tang D, Xiao W, Xing L, Zhu H, Wang D, SadowaY DR (2013) Capture and electrochemical conversion of CO2 to value-added carbon and oxygen by molten salt electrolysis. Energy Environ Sci 6(5):1538–1545
Zainulabdeen AA, Hashim FA, Assi SH (2019) Mechanical properties of tin-based babbitt alloy using the direct extrusion technique. IOP Conf Ser Mater Sci Eng 518(3):032031
Acknowledgements
This work was financially supported by Postgraduate Institute of Science, Peradeniya, Sri Lanka. The sample analysis was performed using X-ray diffractometer that is available at modern X-ray diffraction facility of Uva Wellassa University Sri Lanka.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The author has no competing interest to declare that are relevant to the content of this article.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Karunadasa, K.S.P. Structure-stability correlations in terms of microstructure during tin oxidation as examined by in situ high-temperature X-ray powder diffraction. Chem. Pap. 78, 3617–3628 (2024). https://doi.org/10.1007/s11696-024-03333-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11696-024-03333-5