Abstract
This study aims to study the possibility of distinguishing the UV–Vis–NIR spectra of filamentous fungi on a paper substrate from the background. Model samples of five filamentous fungi were used: Alternaria alternata, Aspergillus niger, Cladosporium herbarum, Penicillium chrysogenum, and Trichoderma atroviride. The model samples were cultivated on paper substrates, and two methods, cross-validation (CV) and principal component analysis (PCA), were utilised to compare their spectra with the reference background spectra. The results of the CV analysis indicated that certain combined spectra sets of Cladosporium herbarum, Penicillium chrysogenum, Trichoderma atroviride, Aspergillus niger, and Alternaria alternata, at specific surface concentrations, exhibited two active components, signifying distinguishable differences from the background spectra. Additionally, the score scatter diagrams derived from PCA revealed clusters of samples, further confirming the distinguishability of the filamentous fungi spectra from the background. However, for Trichoderma atroviride, the scatter diagram demonstrated a relatively large scattering of points, impeding the resolution of spectra with a surface concentration of 2 105 cm−2 due to measurement inaccuracies. Based on the combined results of CV and PCA, the study concluded that the lower threshold of measurability for UV–Vis–NIR spectra varied among the different filamentous fungi. For example, Cladosporium herbarum, Penicillium chrysogenum, and Trichoderma atroviride exhibited a threshold around a surface concentration of 2 106 cm−2, while Aspergillus niger had a threshold around 2 105 cm−2, and Alternaria alternata had a threshold around 2 103 cm−2. In summary, this research provides insights into the distinguishability of filamentous fungi spectra on the paper substrate from background spectra using spectral analysis techniques, offering potential applications in fungal identification and characterisation.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The invention of paper is closely connected with the development of human civilisation. A large percentage of the world’s valuable historical heritage is recorded on paper carriers and stored in conditions that create favourable conditions for the growth and proliferation of microorganisms. The most important microorganisms threatening cultural heritage are microscopic filamentous fungi. Filamentous fungi found in archives, libraries, and other memory institutions are frequently from the genera Penicillium, Aspergillus, Cladosporium, Chaetomium, and Trichoderma (Pinheiro et al. 2019).
Paper, as an organic substrate with hygroscopic character, provides filamentous fungi with nutrients that serve as a source of energy. Fungi can cause severe damage to paper-based heritage objects, associated with various chemical, physical or aesthetic changes. Physical processes of fungi-induced degradation occur mainly under the influence of hyphal growth or by forming fruiting bodies on the contaminated object. The formation and spreading of mycelium over the object’s surface changes the visual properties, ultimately affecting the aesthetic value (Savković et al. 2022). Certain filamentous fungi are characterised by producing of paper-staining pigments or weak organic acids that can induce discolouration (Dunca and Elena 2014). The production of organic acids lowers the pH of the paper, which can lead to acid hydrolysis (Híreš 2015). Another consequence of the degradative activity of fungi might be so-called foxing—the formation of isolated reddish-brown spots (Szulc et al. 2018). Thanks to their enzymatic activity, structural alternations associated with the degradation of the main component of paper—cellulose fibres—occur, leading to the loss of the characteristic structure of the paper and changes in its properties. Paper progressively loses its strength, becomes brittle, and even object destruction might occur (Dunca and Elena 2014; Híreš 2015). Besides, filamentous fungi can participate in degrading historical glues, oil binders or other essential added components (e.g. adhesives, antioxidants, etc.) (Sterflinger and Piñar 2013; Dunca and Elena 2014).
Before any intervention by the conservator, the cultural heritage objects should be diagnosed to evaluate their current state, including assessment of biological damage. Knowing a complete set of information about the microbial communities contaminating artefacts is necessary. Proper detection, characterisation, and identification of microflora using a suitably chosen combination of methods are crucial for appropriately assessing potential threats to cultural heritage artefacts (Savković et al. 2019). Unfortunately, the numbers of standard approaches used for detecting and identifying microorganisms are time-consuming, destructive and not very simple. Spectroscopic techniques are a promising, non-destructive, fast and simple alternative.
Fischer et al. (2006) and Shapaval et al. (2012) present methods that can serve as a basis for differentiation and strain characterisation of various species of filamentous fungi based on Fourier transform infrared spectroscopy (FTIR). Lecellier et al. (2014) showed that FTIR spectroscopy is promising for routine fungal identification. A study by Salman et al. (2010) shows differences between examined fungi genera using FTIR ATR spectroscopy. Surface-enhanced Raman spectroscopy (SERS) was employed to discriminate fungi found in biofilms formed on earthen architecture walls in the region known as Paraíba Valley (or São Paulo Historical Valley) (Fazio et al. 2018).
UV–Vis–NIR FORS (fibre optic reflectance spectroscopy) is a helpful tool as the technique has several advantages: (1) it requires short times of analysis (a good quality spectrum can be obtained within a few seconds); (2) easy to use; (3) non-destructive and non-invasive technique—no physical or chemical alteration to the analysed area; (4) availability of not very expensive instrumentation (Aceto et al. 2014); (5) instruments do not set any limitation to the size or shape of analysed objects; and (6) portability of instrumentation (Analytical Methods Committee AMCTB No 75 2016). UV–Vis–NIR spectroscopy has been increasingly used in cultural heritage protection in recent years. Its variant with optical fibres is becoming one of the most widely used tools for analysing heritage objects and materials (Analytical Methods Committee AMCTB No 75 2016), as it enables the acquisition of spectral data directly in collection-keeping institutions. The method has already been used for the identification and characterisation of pigments (Garofano et al. 2016), the study of degradation mechanisms, and differentiation between different textiles (wool, silk, cotton, and bast) (Analytical Methods Committee AMCTB No 75 2016), characterisation of discolouration in historical plastic objects (Angelin et al. 2020), identification of iron-gall inks (Gál et al. 2013), identification of corrosion products on bronze artefacts (Liu et al. 2021), and many others. Nevertheless, it has rarely been applied in studying the microbial contamination of historical artefacts.
This research aimed to investigate the potential of portable UV–Vis–NIR fibre optics reflectance spectroscopy as a tool for the non-destructive study of filamentous fungi contaminating paper and heritage paper documents.
Experimental
Microscopic filamentous fungi
Fungi cultures of Alternaria alternata, Aspergillus niger, Cladosporium herbarum, Penicillium chrysogenum and Trichoderma atroviride were cultivated on malt extract agar (MEA; Biolife, Italy) at room temperature for 5–10 days. Fungi cultures were selected based on a literature search as representatives of the genera most often found in the environment of memory institutions (Skóra et al. 2015; Koul and Upadhyay 2018; Pinheiro et al. 2019).
Paper substrate
Whatman® Grade 1 Chr paper made from pure cellulose produced entirely from the highest quality cotton linters, with a basic weight of 87 g m−2 and without additives, was used. Whatman papers with 3.5 × 3.5 cm square size were sterilised by autoclaving (120 °C, 120 kPa, 20 min).
Sample preparation
After cultivating selected fungi, a few millilitres of sterilised distilled water was poured into the Petri dish. Conidia were released using a sterile L-shaped spreader, and the fungal suspension was filtered through a sterile gauze three times. The concentration of conidia in the suspension (the total number of cells per ml) was determined using a Bürker chamber. Subsequently, inocula with different conidia concentrations in 1 ml of the liquid medium (Table 1) were prepared. In the case of Alternaria alternata, inoculum with a concentration of 109 conidia per 1 ml of the liquid medium could not be prepared. It is related to the structure of the conidia and the formation of fewer conidia. Therefore, 10 µl of each inoculum was applied to Whatman papers. Three parallel samples of each concentration were prepared. Samples were left to air dry in sterile Petri dishes for 24 h. Finally, the surface concentrations of samples were calculated (Table 1).
Spectral measurements and processing of spectral data
An optical fibre UV–Vis–NIR spectrometer Ocean Optics HR 4000 with DH-2000-BAL source was used to measure the UV–Vis–NIR spectra. The light source is a combined deuterium-halogen source that provides light in the wavelength range of 200–1100 nm. The OceanView programme was used to analyse and process data with the following settings: integration time, automatic; the number of scans, 5; boxcar, 10.
The raw spectral data were interpolated in the range 300–1000 nm (140 points), and reflectance values R (λ) were transformed to optical density values D (λ):
The optical density spectra are generally regarded as more suitable for the methods of factor analysis using linear algebra (Čeppan et al. 2003). Also, each spectrum was normalised in the interval 0–1.
As reference samples, 10 µl of distilled water on the Whatman® papers were applied and processed in the same way as sample spectra, were used.
Two procedures based on the factor analysis approach—cross-validation (CV) and two-dimensional scatter diagrams of factor scores obtained by principal component analysis (PCA)—were used to evaluate whether the spectra of the model systems differ from the background spectra.
The cross-validation method determines the number of active components in a set of spectra (Pelikan et al. 1994; Malinowski 2002). Briefly, in CV, single spectral vector is removed from the spectral matrix, and the resulting submatrix is submitted to the factor analysis successively with the number of factors from 1 to n (n is the number of spectra in the submatrix). The removed spectral vector is successively projected on factor subspaces of this submatrix size 1 to n. The above procedure is repeated until all set spectra (all columns of the spectral matrix) have been projected. The match between the original and projected spectra becomes closer with increasing factors used in the projection. Reaching the arbitrary value of the chosen criterion (e.g. the standard deviation between the original and projected spectra, called standard deviation of cross-validation SDOCV) for the match, which does not change significantly when more factors are added, indicates that the proper number of active components has been attained. Details of CV can be found elsewhere. (Gál et al. 2013).
The principal component analysis method uses a matrix decomposition of spectral data and a corresponding reduction in the number of variables to describe the spectra of the studied samples in a reduced space. The PCA allows to detection of the tendency of samples with comparable properties to cluster into groups and reveal the latent internal structure of the data. The results of the PCA method—two-dimensional score scatter diagrams—allow observing the distribution of samples into clusters. It is important to note that samples located in one cluster are similar to each other, and at the same time, they are different from samples located in other clusters.
Results and discussion
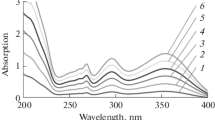
Figure 1 shows the normalised reference spectra of background samples. The standard deviation of these spectra is 0.04.
Figures 2, 3, 4, 5 and 6 show the average normalised spectra of model samples of five filamentous fungi. Each average spectrum was obtained from the set of three spectra. As mentioned above, the normalised spectra of model spectra of filamentous fungi (three spectra of each model) were compared with the set of reference spectra using two procedures based on the factor analysis approach—cross-validation and two-dimensional scatter diagrams of factor scores obtained by principal component analysis. In both methods, the spectra of sets are arranged column-wise to the matrices.
The CV was used to determine the number of active components in the combined spectra sets consisting of the spectra of model samples of given surface concentration and the reference background spectra. Indication of two active components by CV in such combined spectra set means that the spectra of model filamentous fungi differ from the spectra of the background, and an indication of only one active component, on the other hand, means that the spectra of the model filamentous fungi cannot be resolved from the spectra of background. The results of the CV analysis are in Tables 2, 3, 4, 5, and 6.
The PCA analysis is most readily achieved by plotting the component scores of the spectral matrix PC1 vs PC2, which shows the collection of samples into the clusters. Generally, the samples located close to each other in a given cluster are similar and differ from samples from any other cluster. Score scatter diagrams of spectra sets consisting of spectra of model samples of given filamentous fungi of all surface concentrations and the reference background spectra are shown in Figs. 7, 8, 9, 10, and 11.
As can be seen from Tables 1, 2, 3, 4, and 5, CV analysis indicates two active components for combined spectra sets of samples of Cladosporium herbarum, Penicillium chrysogenum, and Trichoderma atroviride with surface concentrations 2 107 cm−2 and 2 106 cm−2, for combined spectra set of samples Aspergillus niger with surface concentrations 2 107 cm−2, 2 106 cm−2, and 2 105 cm−2 and combined spectra sets of samples of Alternaria alternata with surface concentrations 2 106 cm−2, 2 105 cm−2, 2 104 cm−2, and 2 103 cm−2. Clustering of points in score scatter diagrams (Figs. 7, 8, 9, 10 and 11) that correspond to the samples spectra of studied filamentous fungi outside the points corresponding to the background spectra indicates that the spectra of the samples of Cladosporium herbarum, Penicillium chrysogenum and Trichoderma atroviride with surface concentrations 2 107 cm−2 and 2 106 cm−2, the spectra of Aspergillus niger with surface concentrations 2 107 cm−2, 2 106 cm−2, and 2 105 cm−2 and the spectra of Alternaria alternata with surface concentrations 2 106 cm−2, 2 105 cm−2, 2 104 cm−2, and 2 103 cm−2 are distinguishable from the background spectra.
On the score scatter diagram of Trichoderma atroviride (Fig. 10), the resolution of the spectra of the samples of Trichoderma atroviride with a surface concentration of 2 105 cm−2 from the background spectra is prevented by a relatively large scattering of points due to the greater inaccuracy of the spectral measurements in this case.
Based on the results of both used methods, it can therefore be concluded that the lower threshold of measurability of the UV–Vis–NIR spectra of conidia of filamentous fungi of Cladosporium herbarum, Penicillium chrysogenum, and Trichoderma atroviride is in the region of surface concentration 2 106 cm−2, for spectra of Aspergillus niger is in the region of surface concentrations 2 105 cm−2 and for the spectra of Alternaria alternata in the region of surface concentration 2 103 cm−2.
Conclusions
The study analysed the spectral data of background samples and model samples of five filamentous fungi. The average normalised spectra of the model samples were obtained from three spectra each. Cross-validation (CV) and principal component analysis (PCA) were used to compare the model spectra with the reference background spectra. CV analysis determined the number of active components in the combined spectra sets. Two active components indicated that the model filamentous fungi spectra differed from the background spectra, while one active component indicated that they could not be distinguished. PCA analysis involved plotting the component scores (PC1 vs PC2) to observe sample clusters and similarities.
The CV analysis results indicated two active components for certain combined spectra sets of Cladosporium herbarum, Penicillium chrysogenum, Trichoderma atroviride, Aspergillus niger, and Alternaria alternata at specific surface concentrations. The score scatter diagrams (Figs. 7, 8, 9, 10 and 11) showed that the spectra of the studied filamentous fungi samples were distinguishable from the background spectra. However, the score scatter diagram of Trichoderma atroviride indicated a relatively large scattering of points, which hindered the resolution of spectra with a surface concentration of 2 105 cm−2 from the background spectra due to measurement inaccuracies. Based on both methods used (CV and PCA), it was concluded that the lower threshold of measurability for UV–Vis–NIR spectra of conidia varied among the different filamentous fungi: Cladosporium herbarum, Penicillium chrysogenum, and Trichoderma atroviride had a threshold around a surface concentration of 2 106 cm−2, Aspergillus niger had a threshold around 2 105 cm−2, and Alternaria alternata had a threshold around 2 103 cm−2. The standard deviations listed in Tables 2, 3, 4, 5, and 6 give the method’s reproducibility. This work represents the first step in developing a comprehensive method for distinguishing microbial contamination of paper by filamentous fungi. With the acquired knowledge, evaluating the method’s applicability for other species is impossible, and it is first necessary to investigate other species contaminating the cultural heritage. In the subsequent work, it would be appropriate to expand the range of investigated filamentous fungi and to gradually include the spectra of real vital and devitalised samples of microbially contaminated paper from archives and collections into the database.
References
Aceto M, Agostino A, Fenoglio G et al (2014) Characterisation of colourants on illuminated manuscripts by portable fibre optic UV-visible-NIR reflectance spectrophotometry. Anal Methods 6:1488–1500. https://doi.org/10.1039/C3AY41904E
Analytical Methods Committee AMCTB No 75 (2016) UV-visible-NIR reflectance spectrophotometry in cultural heritage: background paper. Anal Methods 8:5894–5896. https://doi.org/10.1039/C6AY90112C
Angelin EM, Cucci C, Picollo M (2020) UV-Vis-NIR reflectance spectroscopy: its application to the study of plastic heritage. In: Colour and colorimetry multidisciplinary contributions, vol XVIB. pp 115–121
Čeppan M, Fedak J, Dvonka V et al (2003) Factor spaces of spectra sets of color prints. J Imaging Sci Technol 47(2):171–184
Dunca SI, Elena A (2014) Study of the contaminating microbiota of old paper supports. Eur Sci J 3:237–251
Fazio AT, López MM, Temperini MLA, de Faria DLA (2018) Surface enhanced Raman spectroscopy and cultural heritage biodeterioration: fungi identification in earthen architecture from Paraíba Valley (São Paulo, Brazil). Vib Spectrosc 97:129–134. https://doi.org/10.1016/J.VIBSPEC.2018.06.002
Fischer G, Braun S, Thissen R, Dott W (2006) FT-IR spectroscopy as a tool for rapid identification and intra-species characterization of airborne filamentous fungi. J Microbiol Methods 64:63–77. https://doi.org/10.1016/J.MIMET.2005.04.005
Gál L, Ceppan M, Reháková M et al (2013) Chemometric tool for identification of iron-gall inks by use of visible-near infrared fibre optic reflection spectroscopy. Anal Bioanal Chem. https://doi.org/10.1007/s00216-013-7333-z
Garofano I, Perez-Rodriguez JL, Robador MD, Duran A (2016) An innovative combination of non-invasive UV–Visible-FORS, XRD and XRF techniques to study Roman wall paintings from Seville, Spain. J Cult Herit 22:1028–1039. https://doi.org/10.1016/J.CULHER.2016.07.002
Híreš O (2015) Mikrobiologická degradácia knižničných a archívnych materiálov. Prejavy mikrobiologickej degradácie knižničných a archívnych materiálov. 2.časť. Knižnica 16:20–22
Koul B, Upadhyay H (2018) Fungi-mediated biodeterioration of household materials, libraries, cultural heritage and its control. In: Fungi and their role in sustainable development: current perspective, pp 597–615. https://doi.org/10.1007/978-981-13-0393-7_32/TABLES/2
Lecellier A, Mounier J, Gaydou V et al (2014) Differentiation and identification of filamentous fungi by high-throughput FTIR spectroscopic analysis of mycelia. Int J Food Microbiol 168–169:32–41. https://doi.org/10.1016/J.IJFOODMICRO.2013.10.011
Liu W, Li M, Wu N et al (2021) A new application of Fiber optics reflection spectroscopy (FORS): Identification of “bronze disease” induced corrosion products on ancient bronzes. J Cult Herit 49:19–27. https://doi.org/10.1016/J.CULHER.2021.03.007
Malinowski ER (2002) Factor Analysis in Chemistry, 3rd edn. Wiley, New York
Pelikan P, Ceppan M, Liska M (1994) Applications of numerical methods in molecular spectroscopy. Taylor & Francis, Milton
Pinheiro AC, Sequeira SO, Macedo MF (2019) Fungi in archives, libraries, and museums: a review on paper conservation and human health. Crit Rev Microbiol 45:686–700. https://doi.org/10.1080/1040841X.2019.1690420
Savković Ž, Stupar M, Unković N et al (2019) In vitro biodegradation potential of airborne Aspergilli and Penicillia. Sci Nat 106. https://doi.org/10.1007/s00114-019-1603-3
Savković Ž, Stupar M, Unković N et al (2022) Fungal deterioration of cultural heritage objects. In: Biodegradation technology of organic and inorganic pollutants. https://doi.org/10.5772/intechopen.98620
Shapaval V, Schmitt J, Møretrø T et al (2012) Characterization of food spoilage fungi by FTIR spectroscopy. https://doi.org/10.1111/jam.12092
Skóra J, Gutarowska B, Pielech-Przybylska K et al (2015) Assessment of microbiological contamination in the work environments of museums, archives and libraries. Aerobiologia (bologna) 31:389–401. https://doi.org/10.1007/s10453-015-9372-8
Sterflinger K, Piñar G (2013) Microbial deterioration of cultural heritage and works of art—tilting at windmills? Appl Microbiol Biotechnol 97:9637–9646. https://doi.org/10.1007/S00253-013-5283-1/FIGURES/4
Szulc J, Otlewska A, Ruman T et al (2018) Analysis of paper foxing by newly available omics techniques. Int Biodeterior Biodegradation 132:157–165. https://doi.org/10.1016/J.IBIOD.2018.03.005
Acknowledgements
This work was funded by the Scientific Grant Agency of The Ministry of Education, Science, Research and Sport of the Slovak Republic VEGA, Project No 1/0602/19. This work was supported by the Slovak Research and Development Agency under the contract No. APVV 20-0410.
Funding
Open access funding provided by The Ministry of Education, Science, Research and Sport of the Slovak Republic in cooperation with Centre for Scientific and Technical Information of the Slovak Republic. Agentúra Ministerstva Školstva,Vedy,Výskumu a Športu SR (Grant no. 1/0602/19); Agentúra na Podporu Výskumu a Vývoja, (Grant no. APVV 20-0410).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
On behalf of all authors, the corresponding author states that there is no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Gál, L., Paračková, P., Kaliňáková, B. et al. Microbial contaminated paper substrate: UV–Vis–NIR spectra of model systems. Chem. Pap. 78, 2603–2611 (2024). https://doi.org/10.1007/s11696-023-03265-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11696-023-03265-6