Abstract
In the present study, a nanocomposite was prepared for the removal of dye from the aqueous phase. In this regard, multi-walled carbon nanotubes (MWCNTs) were anchored on the stalk of Solanum melongena (SMB) to obtain a robust adsorbent with the capacity to eliminate reactive blue 19 (RB19) using the batch adsorptive processes. Solanum melongena stalk decorated with MWCNTs (SMC) and SMB were characterized using Thermogravimetric analysis (TGA), Brunauer–Emmett–Teller (BET), Field emission scanning electron microscopy (FESEM) and Fourier transform infrared spectroscopy (FTIR) analysis. With the exception of solution pH, an increase in contact time, adsorbent dose, initial RB19 concentration, and solution temperature were noticed to elevate the uptake potential of SMB and SMC. Kinetic experimental data for SMB and SMC were consistent with the pseudo-second-order and Elovich model, respectively. The experimental isotherm data obtained for SMB and SMC were best expressed by Freundlich and Langmuir models, respectively. After the fifth adsorption–desorption cycle, SMC exhibited 52% of adsorption efficiency. Hence, SMC can be an auspicious candidate for the efficient adsorption of RB19.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Pollution of water bodies by various dyes is becoming a global problem for mankind. An alarming situation of extreme dye toxicity has been reported in various parts of the world (Hashemi and Kaykhaii 2022). The unruly behaviour of these dyes could be due to the increasing discharge of untreated coloured effluents from a source such as textile, paper, painting, cosmetic, plastic and cell staining industries amongst others. Meanwhile, the high volume of dye in the environment could be responsible for the incessant toxicity attack on both aquatic organisms and man (de Luna et al. 2014). The easy accumulation and nonbiodegradable nature of dyes showcase the impending danger of dye contaminants (Jawad et al. 2022a). An increase in the amount of dyes discharged into the water bodies, increases the chemical and biological oxygen demand of the water bodies, on the other hand, the penetration of sunlight is reduced and aquatic photosynthetic activities impede. Dyes as a water contaminant, have showcased the capacity to be poisonous and the tendency to initiate genetic mutation, cardiovascular disease, mental disorders, cancer, skin allergies, liver and kidney damage, tumours and jaundice, which causes serious impairment to human health (Mate and Mishra 2020; Wang et al. 2020; Yao et al. 2020).
Reactive Blue 19 (RB19) is a water-soluble azo dye (anionic dye) consisting of anthraquinone chemical moiety and it is used for imparting colouring in textile industries (Le et al. 2011; Lee 2003). The high resistance of RB19 to chemical oxidation confers on RB19 a longer water retention time, hence, it is imperative to employ effective techniques for the elimination of RB19 from the water bodies.
Subsequently, various treatment techniques such as coagulation (Shi et al. 2007), anaerobic/aerobic biological treatment (Koupaie et al. 2011), membrane filtration (Hou et al. 2020), electrochemical treatment (Yang et al. 2021), flocculation (Mcyotto et al. 2021), advanced oxidation processes (Nidheesh et al. 2018), photocatalysis (Mahmoodi et al. 2019; Peng et al. 2017), and adsorption (Ahmad et al. 2014; Doğan et al. 2009; Dutta et al. 2021) have been deployed to regulate the dye levels in industrial effluents before discharging. Among the aforementioned wastewater treatment techniques, adsorption has demonstrated promising potential to sequester RB19 from dye-contaminated water. The benefit of this water treatment technique (adsorption) include; high removal efficiency, flexible operation, low cost of operation, ubiquitous adsorbent, ability to be reused and high removal efficiency at low adsorbate concentrations (Dotto and McKay 2020). In the application of this technique, activated carbon (AC) has been proposed as a competent adsorbent for pollutant sequestration. However, the high cost of AC production and poor regeneration tendencies calls for the design of cheap and supper-efficient adsorbent. In order to address these issues, several inexpensive synthetic and natural materials, such as nano-carbon (Liang et al. 2022), watermelon rind (Lakshmipathy and Sarada 2016), MgONPs (Moussavi and Mahmoudi 2009; Nga et al. 2013), chitosan/MgO/Fe3O4 (Jawad et al. 2022b), fly ash and bottom ash (Aarfane et al. 2014), zeolite (Wang et al. 2006), orange peel (Lazim et al. 2015), Bifurcaria bifurcate (Bouzikri et al. 2020), chitosan (Ramazani et al. 2018), spent tea leaves (Lazim et al. 2015), kaolin clay composite (Jawad et al. 2020), bentonite (Tahir and Rauf 2006) hydrogel (Liu et al. 2018), and Moroccan date pits (Badri et al. 2018) amongst others have been used for dye removal. Solanum melongena (eggplant) is an annual plant that is extensively produced in China. The stalk of solanum melongena (SMB) is often discarded and this results in environmental challenges. The SMB are fibrous, hence, may sustain essential adsorptive functional groups that will present SMB as a green adsorbent for RB19 remediation. A considerable impetus has been given recently to the application of multi-walled carbon nanotubes (MWCNTs) as adsorbent/adsorbent modifiers. This is attributed to their large specific surface areas, light density, good porosity and hollow structure. Besides these physical characteristics, MWCNTs have the capacity to trap water contaminants via pore entrapment, π–π interaction and electrostatic interaction. Hence, the application of MWCNTs as a modifier for SMB may enhance the uptake potential of SMB for RB19.
In this study, a nanocomposite fabricated from functionalized MWCNTs and SMB will be employed for the remediation of RB19. The adsorbents (SMC and SMB) will be characterized using different spectroscopic techniques and thereafter employed for batch adsorption of RB19. A possible mechanism for the adsorption of RB19 by SMC was proposed. Essential factors of adsorption such as solution pH, contact time, and initial RB19 concentration and solution temperature will be used to optimize the uptake capacity of the nanocomposite. The adsorption thermodynamics, kinetics and regeneration processes of the nanocomposite (SMC) and SMB were also investigated.
Experimental
Material and chemicals
Analytical grade sodium hydroxide (99.99%), nitric acid (98%), Reactive Blue-19 dye (99.99%), hydrochloric acid (99.9%), sulfuric acid (98%), and sodium chloride (> 95%) were acquired from Sigma-Aldrich and used without supplementary purification. On the contrary, multi-walled carbon nanotubes having a length of 10 to 20 m (average length 17 m) and an exterior diameter of 30 to 50 nm (average diameter 39 nm) were acquired from the same source and purified before use.
Preparation of biomass
Solanum melongena was obtained from the Ahia Eke community market, Umuahia, Abia State, Nigeria (5°30′43.0"N and 7°31′48.0" E). The stalk was detached and washed with distilled water. Thereafter, the stalk was air-dried for eight weeks, reduced into a fine powder, and kept in an airtight container for future use.
Preparation of composites sample (SMC)
Multi-walled carbon nanotubes that have been acid-functionalized (f-MWCNTs) were obtained using the method reported by Amaku’s et al. (2022) (Amaku et al. 2022). Briefly, 0.5 g of MWCNTs were treated with 25 cm3 of 6 M HCl for 6 h under constant stirring at room temperature. The mixture was diluted, filtered, and dried at 120 °C. Thereafter, the procedure was repeated using 6 M HNO3. About 0.5 g of the obtained product was treated with 50 cm3 of an acid mixture (nitric acid-sulfuric 3:1 (v/v)) for 12 h. The mixture was diluted, filtered, and washed to neutral under vacuum. The acid-treated MWCNTs were then dried and stored for further use. Thereafter, a beaker containing 2 g of SMB and 30 cm3 of deionized water was contacted with 0.5 g of functionalized multi-walled carbon nanotubes (f-MWCNTs), and the mixture was stirred for 12 h. Subsequently, the mixture (f-MWCNTs/SMB) was treated with 2.5 cm3 of glutaraldehyde. The mixture was vacuum oven-dried for 72 h at 45 °C, crushed and kept in an airtight container for further use and labelled as SMC.
Batch adsorption
The capacity of SMB and SMC to sequester RB19 from simulated wastewater was assessed via the adsorptive method. The stock solution (1000 mg dm−3) of the adsorbate was prepared by accurately weighing 1 g Reactive Blue-19 powder into 1 dm3 of deionized water. The working solution for further study was prepared from the stock solution by making use of serial dilution. The working solutions employed in the batch adsorption study were adjusted to desired solution pH using 0.1 mol dm−3 NaOH, or 0.1 mol dm−3 HCl. The batch adsorption of RB19 was accomplished by contacting SMB or SMC (0.05 g, 120 rpm) with RB19 solution (100 mg dm−3, 25 cm3) in a 100 cm3 stoppered glass bottle for 1440 min at room temperature. The mixture was filtered, and the residual concentration of RB19 was assessed utilizing ultraviolet–visible (UV–vis) spectrophotometry (λmax = 594 nm). The effects of cardinal adsorptive factors such as initial RB19 concentration, adsorbate pH, adsorbent dose, agitation time and solution temperature were also examined. The uptake capacity (mg g−1) and the uptake efficiency (%) of RB19 were appraised by making use of Eqs. (1) and (2), respectively:
where Ci is the initial RB19 concentration (mg dm−3), Ceq is the concentration at equilibrium (mg dm−3) of RB19, m is the mass (g) of SMB or SMC, and V is the volume of RB19 solution (dm3).
Kinetic and isotherms studies
The rate of RB19 removal by SMB or SMC was assessed using batch adsorption techniques. About 0.05 g of SMB or SMC was contacted with RB19 solution (25 cm3, 100 mg dm−3 adjusted to pH 3) in a stoppered glass bottle (100 cm3). The mixture was contacted for varied time intervals ranging from 5 to 1440 min. Data acquired from the contact time experiment were fitted into pseudo-second order, Morris-Weber intraparticle diffusion models, Elovich, and pseudo-first-order kinetic models (Ho and McKay 1999; Lagergren 1898a; Weber Jr and Morris 1963). A similar experimental condition with variation in the initial RB19 concentration (10 to 100 mg dm−3) was used to investigate the RB19 concentrations in the liquid phase at equilibrium and the amount of RB19 in the solid adsorbent phase at temperatures 295 K, 303 K, 308 K and 318 K. The experimental data obtained from the initial RB19 concentration was fixed to Freundlich and Langmuir isotherms (Freundlich 1907; Langmuir 1916). The equations of the aforementioned models were displayed in Tables 1 and 2.
Regeneration studies
To validate the recyclability of SMB and SMC, adsorption-regeneration-adsorption experiments were conducted. In the adsorption step, 50 cm3 stoppered glass bottles containing 0.5 g of SMC or SMB and 250 cm3 of RB19 solution (100 mg dm−3; pH 3) were contacted for 1440 min. The mixture was filtered and analysed. The used sample was cleaned and dried. Thereafter, RB19-loaded adsorbents (0.05 g) were then agitated with 25 cm3 acetonitrile at room temperature for 20 min and the amount of desorbed RB19 was calculated. The percentage uptake in the next cycle was estimated by making use of Eq. (2).
Results and discussion
Field emission scanning electron microscopy (FESEM) was used to assess the pore structure and surface morphology of SMB and SMC. Figure 1a, b shows the FESEM micrograph acquire for SMB and RB19-loaded SMB. SMB was noticed to exhibit a sheetlike and irregular-shaped fragment morphology. Meanwhile, after the adsorption of RB19 on SMB, the adsorbent was observed to have a smooth surface, indicating successful fixation of the RB19 onto the surface of SMB. On the other hand, a long interwoven cylindrical material that is sandwiched with lumps was noticed for the as-prepared (SMC) and spent nanocomposite (SMC-RB19) (see Fig. 1c, d. The FESEM micrograph SMC-RB19 was noticed to have a smooth surface with some degree of charging, this indicates consistency in the surface coverage of the SMC by the RB19 (see Fig. 1d).
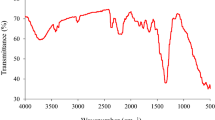
FTIR spectra of SMB, SMC and SMC-RB19 a obtained as displayed in Fig. 2. The spectra showed the presence of chemical moieties that are common to SMB and SMC, including RB19 loaded composite. Meanwhile, peaks at 3356 cm−1, 2890 cm−1, 1607 cm−1, 1352 cm−1 and 1031 cm−1 were obtained and assigned to hydroxyl (–OH stretching), –CH2 vibration, C = C aromatics, stretching mode of C–O, vibration stretching of aliphatic amines (–C–N), respectively (Omer et al. 2018). The emergence of a new peak at 2100 cm−1 suggests the successful fabrication of the nanocomposite and can be attributed to N = C = S. On the other hand, SMC-RB19 revealed a reduction in the intensities of peaks and a slight shift in bands, this indicates the fixation of RB19 to the surface of the nanocomposite. This corroborates the findings deduced from the SEM micrograph.
The physisorptive characteristics of SMB and SMC were examined using Brunauer, Emmett and Teller (BET) N2 adsorption–desorption method and the Barrett-Joyner-Halenda (BJH) pore-size evaluation technique. As shown in Table 3, the pore diameter, pore volume, and surface area of SMC were noticed to be higher than the values obtained for SMB. This could be attributed to the incorporation of f-MWCNTs as modifiers to the biomass (SMB). The plots of N2 adsorption–desorption isotherms of SMB and SMC revealed a Type IV adsorption isotherm with H3 hysteresis loop formation within the relative pressure (P/P0) > 0.80 and > 0.1 (see Fig. 3). Hence, the SMB and SMC are associated with mesopores characteristics (Sing 1985).
The thermal stability of SMC was examined by making use of the thermogravimetric analysis (TGA) under N2 atmosphere (see Fig. 4). SMC showed a weight loss (~ 6%) below 150 °C could be due to the dehydration of the nanocomposite. Meanwhile, weight loss (~ 38%) within 200 and 375 °C could be due to the thermal breakdown of side groups or the devolatilization of inorganic gasses. The temperature ranges of hemicellulose, cellulose, and lignin breakdown were described as 210–320, 315–400, and 150–900 °C, respectively (Yang et al. 2007). Finally, degradation within 350–600 °C (~ 11% weight loss) may be associated with cellulose or lignin contents of the stalk. Hence, the SMC has demonstrated good stability at elevated temperatures.
Effects of pH
In the adsorptive removal of dyes from wastewater, Solution pH plays a vital role. The surface chemistry of the SMB and SMC including the ionization of the RB19 functional group are often altered by solution pH. Hence, it is important to determine the optimum removal pH prior to adsorption proper. Hence, the influence of solution pH on the removal capacity of SMB and SMC was assessed by varying the initial solution pH value from 1–10. Figure 5 reveals that the uptake capacity of SMB and SMC declined with a decrease in solution pH. As the solution pH decreased from 10 to 3, the uptake capacity of SMB and SMC for RB19 increased from 8.58–18.12 mg g−1 and 34.31–42.88 mg g−1, respectively, suggesting that the protonation of RB19 favours its removal from the aqueous phase. It could also be ascribed to the electrostatic repulsion between the negatively charged surface of SMB and the ionized RB19. This result is consistent with findings obtained from the adsorption of reactive blue 19 dye from aqueous solutions using nanohydroxyapatite adsorbent (Ciobanu et al. 2016). The pHPZC of SMB and SMC were 3.1 and 6.3, respectively (see Fig. 6). This demonstrates that at solution pH levels below and above these values (3.1 and 6.3), the surfaces of the SMB and SMC will be positively and negatively charged, respectively. Consequently, at solution pH below 3, the adsorbents will be positively charged. Hence, electrostatic interaction may be chiefly responsible for the adsorption of RB19 onto SMB and SMC. Besides electrostatic interactions, pore entrapment and hydrophobic interactions may be the key reason for the enhanced removal capacity of SMC. This study demonstrated that the removal of RB19 ions from the aquatic ecosystem is significantly dependent on solution pH.
Adsorption kinetics
The implication of contact time and adsorption kinetics experiment was performed at solution pH 3, 100 mg dm−3 (25 cm3) initial RB19 concentration, 0.05 g dosage and 298 K. Figure 7 demonstrates the correlation between the uptake capacities of SMB and SMC with the contact time. The rapid uptake of RB19 was achieved within 120 min by SMB (18.15 mg dm−3) and SMC (39.98 mg dm−3), respectively (see Fig. 7). The rate of RB19 uptake was noticed to reduce until a plateau was reached, indicating an equilibrium attainment. This could be due to the abundance of the binding site on the surface of the adsorbents at the early stage of the adsorption process which gets reduced as the reaction approaches equilibrium. Insite on the mechanism of adsorption was examined by fitting the data acquired from the contact time experiment into four kinetic models, namely the Weber-Morris intraparticle diffusion, pseudo-first order, Elovich and pseudo-second order (see Fig. 8). The unit adsorption of an adsorbate to a specific binding site is best described by the pseudo-first-order model. On the other hand, pseudo-second order assumed chemisorptive binary adsorption of sorbate onto two active sites on the sorbent surface (Amaku et al. 2021). Elovich kinetic model is known to best express chemisorption adsorption processes. Meanwhile, the Weber-Morris intraparticle diffusion model predicts the rate-controlling step of the removal process.
As shown in Table 4, the least sum of square residual (SSR) of the models was used to choose the model that best expressed the data. According to the findings, the Elovich model best describes the uptake of RB19 by SMC. This shows that chemisorption was predominantly responsible for RB19 removal from the aqueous phase. On the other hand, pseudo-second order was noticed to best express the adsorption of RB19 onto SMB, suggesting binary adsorption of RB19 onto SMB (see Table 2).
Dosage effect on RB19 uptake
As it affects the ability of the adsorbent to extract a standard solution of RB19, the amount of adsorbent used to remove dye from simulated wastewater is crucial to the overall adsorption process. The elimination of RB19 increased with increasing SMB and SMC dose, as demonstrated in Fig. 9a. Dosage of 0.05 g resulted in the removal of more than 50% of RB19; hence, this value was carefully selected as an appropriate condition for further adsorptive optimization. The increased adsorption efficiency may be attributed to an increase in the number of interaction sites when the dosage is increased at a fixed concentration of RB19. On the contrary, the uptake capacity of SMB and SMC declined with increased dosage (see Fig. 9b). Therefore, this phenomenon might be caused by aggregation brought on by an excess amount of adsorbent.
Adsorption isotherm and thermodynamic study
The effect of initial RB19 concentration on SMB and SMC absorption capacity was investigated by altering the RB19 concentration from 10 to 100 mg dm−3. The results acquired are displayed in Fig. 10. The curve showed a fast upsurge in uptake potential for initial concentrations ranging from 10 to 100 mg dm−3. The uptake potential of SMB and SMC increased with increasing initial RB19 concentration, suggesting the existence of attractive forces between the adsorbent's surface (SMB and SMC) and anionic RB19. The curve describing the uptake of RB19 by SMB was observed to sustain an equilibrium plateau at initial concentration > 70 mg dm−3, which may reflect the coverage of the active sites engaged in the adsorption of RB19 (see Fig. 10). On the other hand, increasing uptake capacity with increase in initial RB19 concentration was noticed for SMC and this could be attributed to impart of the modifier (f-MWCNTs). The incorporation of f-MWCNTs induced a large number of active sites, hydrophobic characteristics and unique pores for RB19 entrapment.
To establish the mode of interaction of RB19 with SMB and SMC, adsorption isotherm studies were performed using the data obtained from the initial RB19 concentration experiment. The residual concentration of RB19 and its corresponding sorbent-to-sorbate ratio were used for equilibrium modelling using Langmuir and Freundlich models. Meanwhile, models with the least SSR were assumed to best describe the equilibrium data. The Langmuir model was noticed to best describe the equilibrium data acquired for the interaction of RB19 with the surface of SMC (see Table 5). This indicates a monolayer uptake of RB19 onto the homogeneous surface of SMC which constitutes energetically equivalent identical sites, with no interaction between the adsorbed RB19 molecules (Langmuir 1918). Similar findings were reported for the adsorption of RB19 onto chitosan-grafted porous particles (Jiang et al. 2014) and modified bentonite (Özcan et al. 2007). On the other hand, the RB19-SMB interaction was well described by the Freundlich model with \(n >1\) over the studied temperature range (see Table 3). This shows the heterogeneous surface of SMB involving a multilayer RB19 uptake. The values of n also suggest chemisorptive uptake of RB19 onto SMB. These findings are consistent with the report on the adsorptive removal of RB19 onto arginine-functionalized Fe3O4 nanoparticles (Dalvand et al. 2016) and chitosan/MgO/Fe3O4 biocomposite (Jawad et al. 2022a). Meanwhile, SMC and SMB were noticed to have a maximum removal capacity of 67.83 and 21.63 mg g−1, respectively. Meanwhile, SMC demonstrated better superior removal capacity when compared with other adsorbents (see Table 6). The significance of utilizing MWCNTs as modifying biomass (SMB) can be observed in Table 6. It was also discovered that even after numerous cycles, the maximum adsorption capacity of SMC was near the values reported by other authors.
Thermodynamics and adsorbent reuse
To validate the binding force responsible for the uptake of RB19 onto SMC and SMB, enthalpy (ΔH°), entropy (ΔS°), and Gibbs-free (ΔG°) thermodynamic parameters are estimated using Eqs. 3 and 4.
where T is the solution temperature in Kelvin, R is the universal gas constant (8.314 J mol−1 K−1), and K is a constant calculated from the product of the Langmuir constants qm and b in dm3 mol−1 which was corrected to be dimensionless by multiplying by 1000.
To achieve this, the influence of solution temperature on the uptake of RB19 by SMC and SMB was investigated. It was noticed that at elevated solution temperature, the removal potential of SMC and SMB was enhanced (see Fig. 10). This indicates the endothermic adsorption process (see Table 7). This effect can be ascribed to the formation of new binding sites on the surface of SMC and SMB or the enlarged sorbent pores. The amount of heat evolved in a physical adsorption process is of the same order of magnitude as the heat of condensation., i.e., 2.1–20.9 kJ mol−1, whereas the heat released during a chemisorption process is frequently within a range of 80–200 kJ mol−1 (Huang et al. 2007; Saha and Chowdhury 2011). Therefore, as displayed in Table 7, it seems that the adsorption of RB19 onto SMC and SMB can be attributed to physisorption. Meanwhile, a positive entropy value was obtained for SMC and SMB, respectively, indicating high interface interactions. Negative values of ∆G° were estimated for all the studied temperatures. This indicates spontaneous and favourable uptake of RB19 by SMC and SMB. Hence, the adsorptive removal of RB19 by SMC and SMB was successful.
Mechanism of adsorption
To understand the mechanism responsible for the adsorption of RB-19 onto SMC, the surface functional groups of SMC, pHPZC, the effect of solution pH and the reaction kinetics of the adsorption process were deductively correlated. As observed in Fig. 2, SMC sustained amine, hydroxyl and carbonyl functional groups. These chemical moieties function as good binding sites for adsorbate when ionized. Meanwhile, the implication of solution pH and pHPZC experiments revealed that the uptake of RB19 onto SMC was best in the acidic medium. This indicates that the surface of the adsorbents will be protonated (amino (–NH3+), hydroxyl (–OH2+) groups). Hence, electrostatic interaction between the positively charge chemical moieties on the surface of SMC and negatively charged (–SO3−) groups of the RB-19 dye. On the other hand, the enhanced pore volume and pore diameter of SMC (see Table 3) will play a vital role in the uptake of RB19 by SMC via pore entrapment of the acid dyes. This is further justified by the contribution of intraparticle diffusion as a mechanistic approach to dye removal (see Table 5). Another contributing removal route can be the hydrogen bonding between hydrogen atoms on the surface of SMC and the nitrogen or oxygen atoms of the RB19. On the contrary, another interaction can be generated between the aromatic systems of RB19 and the hydrogen of the hydroxyl groups on SMC (Yoshida H-bonding). Finally, the π-electrons on the aromatic ring of RB19 can interact with π-bonds of SMC via π-π interaction. Hence, the adsorption of RB19 onto SMC is predominantly electrostatic. However, H-bonding, pore entrapment, Yoshida H-bonding and π-π interaction are subordinate reaction paths through which RB19 was sequestered by SMC (see Fig. 11). The proposed removal mechanism is in good agreement with the report of other authors (Jawad et al. 2022a; Jiang et al. 2014; Ogunleye et al. 2020).
Reusability of SMB and SMC
The overall value of an adsorbent is a function of its capacity to effectively sequester water contaminants and its tendency to be reused. To establish the aforementioned, the reuse of SMB and SMC after an adsorption–desorption step was executed for five cycles. As displayed in Fig. 12, the removal percentage of SMC and SMB was observed to decline with the increase in the number of cycles and this could be due to the augmented deposition of the reaction product at the adsorption site with an increase in the number of cycles. However, the uptake efficiency of SMC and SMB was above 52% and 27% for the fifth cycle, respectively. This shows that the application of f-MWCNTs as SMB modifier was effective in the elimination of RB19 from the aquatic ecosystem. Hence, SMC has demonstrated robust and unique characteristics for application in environmental remediation practices (Fig. 12).
Conclusion
This study presents the successful synthesis of nanocomposite from the stalk of solanum melongena and MWCNTs. It also investigated the effectiveness of these adsorbents (SMB and SMC) for RB19 capture from simulated wastewater. A comparison of the maximum monolayer uptake potential of SMB (21.63 mg g−1) and SMC (67.83 mg g−1) with other adsorbents revealed the superior nature of SMC. The maximum effectiveness of SMB and SMC was achieved at solution pH 3.0, dosage of 0.05 g and 120 min contact time. Isotherm study reveals that the adsorption of RB19 onto SMB and SMC was best described by Freundlich and Langmuir models, respectively. Meanwhile, the adsorption of RB19 onto SMB and SMC is thermodynamically viable. Thus, SMB and SMC can be scale-up for wastewater treatment practices. The application of SMC in the treatment of wastewater comprising various contaminants of varying chemistry would be the perspective of this study.
Data availability
Data will be supplied upon request from the corresponding author.
References
Aarfane A, Salhi A, El Krati M, Tahiri S, Monkade M, Lhadi E, Bensitel M (2014) Etude cinétique et thermodynamique de l’adsorption des colorants Red195 et Bleu de méthylène en milieu aqueux sur les cendres volantes et les mâchefers (Kinetic and thermodynamic study of the adsorption of Red195 and Methylene blue dyes on fly ash and bottom ash in aqueous medium). J Mater Environ Sci 5:1927–1939
Ada K, Ergene A, Tan S, Yalçın E (2009) Adsorption of remazol brilliant blue R using ZnO fine powder: equilibrium, kinetic and thermodynamic modeling studies. J Hazard Mater 165:637–644
Ahmad MA, Ahmad N, Bello OS (2014) Adsorptive removal of malachite green dye using durian seed-based activated carbon. Water Air Soil Pollut 225:2057
Amaku JF, Ogundare S, Akpomie KG, Ibeji CU, Conradie J (2021) Functionalized MWCNTs-quartzite nanocomposite coated with Dacryodes edulis stem bark extract for the attenuation of hexavalent chromium. Sci Rep 11:1–15
Amaku JF, Olisah C, Adeola AO, Iwuozor KO, Akpomie KG, Conradie J, Adegoke KA, Oyedotun KO, Ighaloi JO (2022) Multiwalled carbon nanotubes versus metal-organic frameworks: a review of their hexavalent chromium adsorption performance. Int J Environ Anal Chem 56:1–23
Asgher M, Bhatti HN (2012) Removal of reactive blue 19 and reactive blue 49 textile dyes by citrus waste biomass from aqueous solution: equilibrium and kinetic study. Can J Chem Eng 90:412–419
Badri N, Zbair M, Sahibed-Dine A, Chhiti Y, Khamliche L, Bensitel M (2018) Adsorption of cationic dyes by waste biomass treated by phosphoric acid. J Mater Environ Sci 9:1636–1644
Bouzikri S, Ouasfi N, Benzidia N, Salhi A, Bakkas S, Khamliche L (2020) Marine alga “Bifurcaria bifurcata”: biosorption of Reactive Blue 19 and methylene blue from aqueous solutions. Environ Sci Pollut Res 27:33636–33648
Chinoune K, Bentaleb K, Bouberka Z, Nadim A, Maschke U (2016) Adsorption of reactive dyes from aqueous solution by dirty bentonite. Appl Clay Sci 123:64–75
Ciobanu G, Barna S, Harja M (2016) Kinetic and equilibrium studies on adsorption of Reactive Blue 19 dye from aqueous solutions by nanohydroxyapatite adsorbent. Arch Environ Prot 25:7892132
Dalvand A, Nabizadeh R, Ganjali MR, Khoobi M, Nazmara S, Mahvi AH (2016) Modeling of Reactive Blue 19 azo dye removal from colored textile wastewater using L-arginine-functionalized Fe3O4 nanoparticles: optimization, reusability, kinetic and equilibrium studies. J Magn Magn Mater 404:179–189
de Luna LA, da Silva TH, Nogueira RFP, Kummrow F, Umbuzeiro GA (2014) Aquatic toxicity of dyes before and after photo-Fenton treatment. J Hazard Mater 276:332–338
Doğan M, Karaoğlu MH, Alkan M (2009) Adsorption kinetics of maxilon yellow 4GL and maxilon red GRL dyes on kaolinite. J Hazard Mater 165:1142–1151
Dotto GL, McKay G (2020) Current scenario and challenges in adsorption for water treatment. J Environ Chem Eng 8:103988
Dutta S, Gupta B, Srivastava SK, Gupta AK (2021) Recent advances on the removal of dyes from wastewater using various adsorbents: A critical review. Mater Adv 2(14):4497–4531
Freundlich H (1906) Over the adsorption in solution. J Phys Chem 57:e470
Freundlich H (1907) Ueber Kolloidfällung und adsorption. Z. Chem Indust Kolloide 1:321–331
Hashemi SH, Kaykhaii M (2022) Azo dyes: sources, occurrence, toxicity, sampling, analysis, and their removal methods, Emerging freshwater pollutants. Elsevier, pp 267–287
Ho Y-S, McKay G (1999) Pseudo-second order model for sorption processes. Process Biochem 34:451–465
Hou J, Chen Y, Shi W, Bao C, Hu X (2020) Graphene oxide/methylene blue composite membrane for dyes separation: Formation mechanism and separation performance. Appl Surf Sci 505:144145
Huang X, Gao N-y, Zhang Q-l (2007) Thermodynamics and kinetics of cadmium adsorption onto oxidized granular activated carbon. J Environ Sci 19:1287–1292
Jawad AH, Abdulhameed AS, Abd Malek NN, Alothman ZA (2020) Statistical optimization and modeling for color removal and COD reduction of reactive blue 19 dye by mesoporous chitosan-epichlorohydrin/kaolin clay composite. Int J Biol Macromol 164:4218–4230
Jawad AH, Rangabhashiyam S, Abdulhameed AS, Syed-Hassan SSA, Alothman ZA, Wilson LD (2022a) Process optimization and adsorptive mechanism for reactive blue 19 dye by magnetic crosslinked chitosan/MgO/Fe3O4 biocomposite. J Polym Environ 30:2759–2773
Jawad AH, Sahu UK, Jani NA, Alothman ZA, Wilson LD (2022b) Magnetic crosslinked chitosan-tripolyphosphate/MgO/Fe3O4 nanocomposite for reactive blue 19 dye removal: Optimization using desirability function approach. Surf Interfaces 28:101698
Jiang X, Sun Y, Liu L, Wang S, Tian X (2014) Adsorption of CI Reactive Blue 19 from aqueous solutions by porous particles of the grafted chitosan. Chem Eng J 235:151–157
Koupaie EH, Moghaddam MA, Hashemi S (2011) Post-treatment of anaerobically degraded azo dye Acid Red 18 using aerobic moving bed biofilm process: enhanced removal of aromatic amines. J Hazard Mater 195:147–154
Lagergren S (1898a) Zur theorie der sogenannten adsorption geloster stoffe. K Sven Vetenskapsak Handl 24:1–39
Lagergren SK (1898b) About the theory of so-called adsorption of soluble substances. Sven Vetensk Handingarl 24:1–39
Lakshmipathy R, Sarada N (2016) Methylene blue adsorption onto native watermelon rind: batch and fixed bed column studies. Desalin Water Treat 57:10632–10645
Langmuir H (1906) The adsorption of gases on plane surface of glass, mica and platinum. J Am Chem Soc 30:1361
Langmuir I (1916) The constitution and fundamental properties of solids and liquids. Part I. Solids. J Am Chem Soc 38:2221–2295
Langmuir I (1918) The adsorption of gases on plane surface of glass, mica and platinum. J Am Chem Soc 40:1361–1403
Lazim ZM, Mazuin E, Hadibarata T, Yusop Z (2015) The removal of methylene blue and Remazol Brilliant Blue R dyes by using orange peel and spent tea leaves. J Teknol 74:369874
Le C, Wu J-H, Li P, Wang X, Zhu N-W, Wu P-X, Yang B (2011) Decolorization of anthraquinone dye Reactive Blue 19 by the combination of persulfate and zero-valent iron. Water Sci Technol 64:754–759
Lee YH (2003) Reductive biotransformation and decolorization of reactive anthraquinone dyes. Ga Inst Technol 45:56895
Liang C, Shi Q, Feng J, Yao J, Huang H, Xie X (2022) Adsorption behaviors of cationic methylene blue and anionic reactive blue 19 dyes onto nano-carbon adsorbent carbonized from small precursors. Nanomaterials 12:1814
Liu L, Wang R, Yu J, Hu L, Wang Z, Fan Y (2018) Adsorption of Reactive Blue 19 from aqueous solution by chitin nanofiber-/nanowhisker-based hydrogels. RSC Adv 8:15804–15812
Mahmoodi NM, Abdi J, Taghizadeh M, Taghizadeh A, Hayati B, Shekarchi AA, Vossoughi M (2019) Activated carbon/metal-organic framework nanocomposite: Preparation and photocatalytic dye degradation mathematical modeling from wastewater by least squares support vector machine. J Environ Manage 233:660–672
Mate CJ, Mishra S (2020) Synthesis of borax cross-linked Jhingan gum hydrogel for remediation of Remazol Brilliant Blue R (RBBR) dye from water: adsorption isotherm, kinetic, thermodynamic and biodegradation studies. Int J Biol Macromol 151:677–690
Mcyotto F, Wei Q, Macharia DK, Huang M, Shen C, Chow CW (2021) Effect of dye structure on color removal efficiency by coagulation. Chem Eng J 405:126674
Moussavi G, Mahmoudi M (2009) Removal of azo and anthraquinone reactive dyes from industrial wastewaters using MgO nanoparticles. J Hazard Mater 168:806–812
Nga NK, Hong PTT, Dai Lam T, Huy TQ (2013) A facile synthesis of nanostructured magnesium oxide particles for enhanced adsorption performance in reactive blue 19 removal. J Colloid Interface Sci 398:210–216
Nidheesh P, Zhou M, Oturan MA (2018) An overview on the removal of synthetic dyes from water by electrochemical advanced oxidation processes. Chemosphere 197:210–227
Ogunleye DT, Akpotu SO, Moodley B (2020) Adsorption of sulfamethoxazole and reactive blue 19 using graphene oxide modified with imidazolium based ionic liquid. Environ Technol Innov 17:100616
Omer A, Khalifa R, Hu Z, Zhang H, Liu C, Ouyang X-k (2018) Fabrication of tetraethylenepentamine functionalized alginate beads for adsorptive removal of Cr (VI) from aqueous solutions. Int J Biol Macromol 125:1221–1231
Özcan A, Ömeroğlu Ç, Erdoğan Y, Özcan AS (2007) Modification of bentonite with a cationic surfactant: an adsorption study of textile dye Reactive Blue 19. J Hazard Mater 140:173–179
Peers A (1965) Elovich adsorption kinetics and the heterogeneous surface. J Catal 4:499–503
Peng Y, Wang KK, Liu T, Xu J, Xu BG (2017) Synthesis of one-dimensional Bi2O3-Bi2O2.33 heterojunctions with high interface quality for enhanced visible light photocatalysis in degradation of high-concentration phenol and MO dyes. Appl Catal B 203:946–954
Ramazani A, Oveisi M, Sheikhi M, Gouranlou F (2018) Natural polymers as environmental friendly adsorbents for organic pollutants such as dyes removal from colored wastewater. Curr Org Chem 22:1297–1306
Saha P, Chowdhury S (2011) Insight into adsorption thermodynamics. Thermodynamics 16:349–364
Shi B, Li G, Wang D, Feng C, Tang H (2007) Removal of direct dyes by coagulation: the performance of preformed polymeric aluminum species. J Hazard Mater 143:567–574
Silva MM, Oliveira MM, Avelino MC, Fonseca MG, Almeida RK, Silva Filho EC (2012) Adsorption of an industrial anionic dye by modified-KSF-montmorillonite: evaluation of the kinetic, thermodynamic and equilibrium data. Chem Eng J 203:259–268
Sing KS (1985) Reporting physisorption data for gas/solid systems with special reference to the determination of surface area and porosity (Recommendations 1984). Pure Appl Chem 57:603–619
Tahir S, Rauf N (2006) Removal of a cationic dye from aqueous solutions by adsorption onto bentonite clay. Chemosphere 63:1842–1848
Wang S, Li H, Xu L (2006) Application of zeolite MCM-22 for basic dye removal from wastewater. J Colloid Interface Sci 295:71–78
Wang B, Yang X, Ma L, Zhai L, Xuan J, Liu C, Bai Z (2020) Ultra-high efficient pH induced selective removal of cationic and anionic dyes from complex coexisted solution by novel amphoteric biocomposite microspheres. Sep Purif Technol 231:115922
Weber W, Morris J (1962) Advances in water pollution research, Proceedings of the first international conference on water pollution research. Pergamon Press Oxford, Oxford, pp 231
Weber WJ Jr, Morris JC (1963) Kinetics of adsorption on carbon from solution. J Sanit Eng Div 89:31–59
Xiao Y, Azaiez J, Hill JM (2018) Erroneous application of pseudo-second-order adsorption kinetics model: ignored assumptions and spurious correlations. Ind Eng Chem Res 57:2705–2709
Yang H, Yan R, Chen H, Lee DH, Zheng C (2007) Characteristics of hemicellulose, cellulose and lignin pyrolysis. Fuel 86:1781–1788
Yang C, Shang S, Li X-y (2021) Fabrication of sulfur-doped TiO2 nanotube array as a conductive interlayer of PbO2 anode for efficient electrochemical oxidation of organic pollutants. Sep Purif Technol 258:118035
Yao X, Ji L, Guo J, Ge S, Lu W, Cai L, Wang Y, Song W, Zhang H (2020) Magnetic activated biochar nanocomposites derived from wakame and its application in methylene blue adsorption. Biores Technol 302:122842
Funding
Open access funding provided by Walter Sisulu University.
Author information
Authors and Affiliations
Contributions
F.J.A. design and implementation the study; R.T. edited, corrected, improved and supervised the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare that there is no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Amaku, J.F., Taziwa, R. Removal of reactive blue 19 from simulated wastewater using Solanum melongena stalk/MWCNTs: thermodynamics, kinetic, equilibrium and regeneration potentials. Chem. Pap. 78, 1251–1263 (2024). https://doi.org/10.1007/s11696-023-03163-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11696-023-03163-x