Abstract
Some of the wound dressings on the market cause skin tearing and bleeding when removed, slowing the healing process. So, it is difficult to treat infected wounds of sensitive skin patients. Herein, antibacterial and biocompatibility self-healing hydrogel loaded with 9-Aminoacridine and kanamycin sulfate were prepared by grafting poly(β-carboxyethyl acrylate-co-acrylamide) onto sodium alginate. The biological assay demonstrated the hydrogels’ good biocompatibility, which showed no harmful effects on normal human melanocyte cells. In addition, the hydrogels had a powerful antibacterial impact on the various bacterial strains utilized in the investigation. From the study of the rheological properties of the prepared hydrogel, it was found that it is a non-Newtonian fluid. These results suggest the possible utilization of the as-prepared hydrogels in the fabrication of wound healing.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Hydrogels are the most significant candidate for wound dressing compared to other materials due to their high water content, elasticity, biocompatibility, adequate flexibility, and physiological sensitivity (Kamoun et al. 2017; Sood et al. 2014). Recently efforts have been dedicated to developing hydrogels with multi-functional properties, such as adhesive, high mechanical and self-healing. On the other hand, due to their distinctive biological activity (such as the hemostatic and antibacterial properties), low immunological response, good biocompatibility, simplicity of physical and chemical modification, the abundance of supplies, and inexpensive cost, polysaccharides are frequently utilized for hemostasis and wound healing (Li et al. 2022b). Alginate is one of these polysaccharides, a structure similar to the native extracellular matrix, and is commonly used to make hydrogel wound dressing (Kamel et al. 2023). Adhesives hydrogel-based-alginate can keep the surrounding region wet, absorb wound exudate, and aid healing. Divalent cation crosslinking (Lazow et al. 2021), Amidation reaction (Froelich et al. 2023), and carboxyl-based chemical modification (Du et al. 2020) are the main preparation methods of this hydrogel. Additionally, in vivo, alginate cannot be biodegraded, so oxidized alginate is utilized to prepare adhesive hydrogel (Cao et al. 2019). Except for divalent cation crosslinking, amidation reaction, and dopamine-modified alginate as the same as oxidized alginate can react with a variety of amino-containing crosslinking agents (such as protein and amino-containing polysaccharides, etc.) to produce adhesives hydrogel by Schiff base reaction (Chen et al. 2018). Wound adhesives hydrogel has varied adhesion strategies to deal with the various tissue surface and environments of different types of wounds. The adhesion mechanisms can be chemical, physical, or bionic adhesion. Chemical adhesion relates to the covalent crosslinking between carboxyl, amino, and thiol (amino acid residues in proteins) of tissue and hydrogel active groups such as, N-hydroxysuccinimide esters (Zhang et al. 2021), aldehyde (Qu et al. 2018), polyphenols (Zhao et al. 2020), and aryl azides (Ono et al. 2000) of hydrogel. Physical adhesion mainly includes hydrogen bond interactions (Zhang et al. 2020), van der Waals forces (Autumn et al. 2002), electrostatic interactions, p–p or cation–p interaction forces (Zhao et al. 2020), electrostatic interactions (Wang et al. 2020), and hydrophobic interaction (Nishiguchi et al. 2020). While bionic adhesion includes mussel adhesion, gecko adhesion, etc. (Li et al. 2022a).
In addition to the adhesive property, self-healing is more attractive due to their healing ability after damage like tissue (Chen et al. 2018). The mechanisms of hydrogel self-healing include non-covalent and covalent bonds such as Schiff bases, hydrogen bonds, coordination bonds, and Diels–Alder reactions (Khattab and Kamel 2022). Also, the cross-linked hydrogels by dynamic bonds lead to self-healing capacities. As the destruction of hydrogel, the internal dynamic cross-linked networks reverse the fracture and reorganize the hydrogels. Wu et al. prepared a fast self-healing hydrogel by mixing polyvinyl alcohol and 3-aminophenylboronic acid grafted alginate. To increase its cold resistance and electrical conductivity, glycerin and inorganic salts were added to the hydrogel (Wu et al. 2020). Lu et al. developed self-healing hydrogel based on dialdehyde alginate/ polyethyleneimine and loaded strontium-ion. The prepared hydrogel could self-heal breaks in the gel and tightly adhere to the wound, solving the problems of skin defect repair and sports rehabilitation. In addition, the slow release of strontium ions from hydrogel quickly heals the wound by promoting angiogenesis and collagen deposition (Lu et al. 2020).
In this study, a mechanically tough self-healing conducting adhesive hydrogel was prepared for potential wound dressing applications. The hydrogels were designed based on sodium alginate grafted poly(β-carboxyethyl acrylate-co-acrylamide), potassium persulfate was employed as initiator, N, N-dimethylbisacrylamide was used as crosslinker. In addition, 9-Aminoacridine and kanamycin sulfate were separately added to the hydrogel mixture following the addition of the crosslinker. The hydrogel formation proceeded via the formation of free radicals on the alginate surface by the persulfate free radicals. The alginate free radicals attack the vinyl double bonds of the monomers, leading to chain propagation and crosslinked with dimethylbisacrylamide.
Materials and methods
Material
Sodium alginate (SA), β-carboxyethyl acrylate (CEA), and kanamycin sulfate (KS) were purchased from Sigma Aldrich, 9-Aminoacrydine (9-AA) was purchased from Merck. Acrylamide (AM) was from Fluka, and N,N-methylenebisacrylamide (MBA) and potassium persulfate (KPS) were purchased from Across. RPMI-1640 medium, fetal bovine serum (FBS), and trypsin–EDTA was purchased from GIBCO™, Grand Island, New York, USA, 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl)- tetrazolium bromide (MTT) was purchased from Bio Basic Inc., Canada. Acridine orange purchased from (oxford Lab Chem.). Normal human skin cell line (HFB-4) was purchased from VACSERA, Egypt.All chemicals used throughout the work were of the highest analytical grade.
Preparation of hydrogel
Hydrogels were prepared according to the method described in reference (El-Sayed et al. 2023). In brief, to 25 mL of 4% alginate solution, 0.060 g KPS was added after removing dissolved oxygen by purging the nitrogen gas into the alginate solution for 10 min. Then, an aqueous miture of 2 g AM and 1 g CEA was neutralized by KOH solution and mixed with the above alginate solution; the pH of the mixture was adjusted to 5.5. After 1 h, an aqueous MBA (0.124 g) was added to the reaction mixture, raised the temperature to 70, and continued stirring for 2 h additional. The formed hydrogel was washed with water, and the unreacted monomers, homopolymer, and alginate were removed by immersing the hydrogel in boiling acetone. The hydrogel was collected and freeze-dried. For drug loading, 9-AA and KS sulfate aqueous solution were added individually onto the hydrogel mixture after adding MBA for 10 min.
For drug loading, an aqueous solution of 9-Aminoacridine hydrochloric salt (25, 50, and 100 mg/5mL H2O) or kanamycin sulfate (50 mg/5mL H2O) was added individually onto the hydrogel mixture after adding MBA for 10 min. The as-prepared hydrogels have the following code names: (S1) the hydrogel without drugs, (S2, S3, and S4) the hydrogel loaded with 9-AA at 25, 50, and 100 mg/1g of SA, (55) the hydrogel encapsulating KS (50 mg/gSA).
Characterization
The FT-IR spectra for SA, S1, S3, and S5 were measured by FT-IR (Nicolet Impact-400 FT-IR spectrophotometer) in the range of 400–4000 cm−1.
Self-healing ability of the hydrogel
The ability of the hydrogel to repair after cutting was determined by longitudinal cutting of one piece of the hydrogel into two pieces. The two pieces were kept in proximity and assembling of the pieces into one piece was achieved with in together, they were able to retain their original shape and gather into one piece within 60–90 s.
Adhesion test
To measure the adhesive strength of hydrogel, Stainless-steel, glass, rubber, and plastic were applied for the adhere test.
Rheological measurement
Rheological measurements of the hydrogel were carried out through a rotational rheometer, MCR 301 Rheometers, Anton Paar, GMbH, Germany. All rheology measurements were made using a 1 mm diameter, and the temperature was controlled at 25 °C. Before testing, samples were left equilibrating for five minutes to allow mechanical equilibrium. Flow curves were made in control rate mode (CR), varying the shear rate (0.1–10 rad/s) at 20 °C.
Electric conductivity measurement
Electrical measurements were performed using stainless steel electrodes and the powerful alpha analyzer described in our previous work. The measuring cell comprises two parallel stainless-steel electrodes separated by a Teflon circular ring of 2mm thickness and 20mm diameter (El-Sayed et al. 2019).
Cytocompatibility assay
MTT assay
Normal human skin cell line (HFB-4) were purchased from VACSERA, Egypt. Cells were allowed to grow in a humidified cell incubator containing 5% CO2 and 95% air at 37 °C with RPMI supplemented with 1% penicillin–streptomycin, 1% non-essential amino acids and 10% fetal bovine serum (FBS). The cells were grown in 96 well plates to ~ 70% confluence and the cells then were treated with along with the DMSO control and incubated for about 24 h. The media were removed and replaced with 120 µL and 30 µL of MTT 3-(4,5-dimethylthiazol-2yl)-2,5-diphenyl tetrazolium bromide solution at 5 mg/ml was added to each well then plates were incubated with for 4 h at 37 °C in a humidified CO2 incubator. After removal of each well supernatant, addition of 100 µL Dimethylsulphoxide (DMSO) was into each well for color development and then the plates were incubated for 10 min under shaking conditions. Finally, the absorbance was measured at 490 nm with a microplate reader (Nelson et al. 2020).
Cytocompatibility using acridine orange staining
Normal human skin cell line (HFB-4) were purchased from VACSERA, Egypt. Different concentrations of Hydrogels were prepared from each group (0.01, 0.02 and 0.03 gm). The concentrations are used for treating previously cultured cells in 48 well plate in a humidified cell incubator containing 5% CO2 and 95% air at 37 °C with RPMI supplemented with 1% penicillin–streptomycin, 1% non-essential amino acids and 10% fetal bovine serum (FBS). The cells reached about 90% confluent when treated with compounds and incubated for 24 h at 37 °C. After treatment, the Hydrogels were discarded then cells monolayers were washed with PBS. The cells were fixed with 10% paraforamaldehyde and stained with 60 µg/ml acridine orange. The cells were examined with ZOE Fluorescent Cell Imager fluorescence microscopy (BIO-RAD,USA) (Thomé et al. 2016).
In vitro wound healing assay
Normal Human skin cells (3 X 105 cells/well) were cultured in 24 well plates and incubated in a humidified cell incubator containing 5% CO2 and 95% air at 37 °C with RPMI supplemented with 1% penicillin–streptomycin, 1% non-essential amino acids and 10% fetal bovine serum (FBS). After incubation, the cell's monolayers were washed twice with PBS. A vertical scratch was applied using 10–100 μL pipette tip across each well. The washing process through PBS helped to remove the detached cells; this was followed by adding low serum fresh medium to each well, and then the image for the scratch was token at zero time. The hydrogel samples S50A and S50K (20 mg), and a mixture from both hydrogels (20 mg each one) were used after sterilization for 30 min under UV. The scratch closure was imaged after 6 h using ZOE Fluorescent Cell Imager fluorescence microscopy (BIO-RAD, USA). The analysis of the scratch width is calculated using the Image J public domain software (Kamel et al. 2023).
Results and discussion
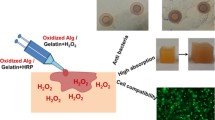
Earlier, we described the preparation and characterization of new hydrogel based on sodium alginate. The hydrogel was prepared by graft polymerization on the surface of SA with poly(β-carboxyethyl acrylate-co-acrylamide). Then, 9-AA was encapsulated into it. Similarly, KS (a broad-spectrum antibiotic) was also physically incorporated into the hydrogel loaded at 5% wt/wt. FT-IR confirmed the structure for the as-prepared hydrogels. Also, the thermal stability and morphological features were determined by TGA and SEM in our previous report. Moreover, the hydrogel swelling study, pH-responsive drug release, antimicrobial activity, and cytocompatibility towards normal lung cell line (VERO) cells were reported (El-Sayed et al. 2023) Fig. 1.
Schematic representation for the hydrogels structures (adapted from (El-Sayed et al. 2023))
Characterization of hydrogels by FT-IR
The grafting of SA and drug loading was characterized by FT-IR spectra (Fig. 2). SA spectrum displayed characteristic absorption bands at 3422 and 3223, 2923, 1608, 1415, 1393, and 1123 cm−1. These peaks are assigned to the inter- and intra-molecular hydrogen bonds, stretching bands for the different −OH groups, aliphatic C–H, asymmetric vibrations of carboxylic and carbonyl groups, stretching (–C–O), and the bending band for –OH groups over the SA chains (El-Sayed et al. 2022a). The appearance of strong absorption bands at 1719, 1656, and 1458 cm−1 in the IR spectrum of S1 hydrogel, which are correlated to the overlapped ester, carboxylic, and amidic carbonyl groups of BMA, poly(ECA), poly(AM), and bending NH respectively proofed the success of graft polymerization. Meanwhile, the two distinctive bands observed at 1485,1406 and 1028 cm−1 represent the bending C–O, C–N, and the deformation vibrations of C–O–C or C–O–H groups. The spectrum of S3 revealed the peaks at 3405 and 3186 cm−1 are due to the OH and NH2 groups, intermolecular H-bonds, and the stretching N–H bonds. The slight red shift of the stretching vibration peaks from 3422 for SA to 3405 cm−1 confirms that hydrogen bonding is the main interaction between 9-AA and the hydrogel network (Jafarigol et al. 2020). In comparison, the stretching bands for the aliphatic and aromatic C–H bonds were recognized in the region between 2931–2846 cm−1. The characteristic sharp and moderate intensity peaks at 1731, 1662, and 1604 cm−1 are assigned to the stretching carboxylate anion and CONH2 groups (Zheng et al. 2023). The stretching C–O and C–N bonds displayed 1550 and 1454 cm−1 peaks. Also, the sulphonic groups' symmetric and asymmetric S–O stretching vibration can be observed at 1388 cm−1 for (υas SO2) and 1172 cm−1 for (υsym SO2), respectively. The stretching absorption band at 1110 cm−1 is for the stretching –C–O bond. For the S5 spectrum, a similar assignment is applied for the peaks at 3409, 3215, 2919–2849, 1733, 1654, 1608, 1575, 1462, 1388, 1171, and 1049 cm−1, respectively (El-Sayed et al. 2022c).
FTIR-spectra of SA, S1, S50K, and S25A, (adapted from (El-Sayed et al. 2023))
Self-healing ability of the hydrogel
Traditional hydrogel dressings cannot self-heal, and external mechanical forces quickly damage them. However, the self-healing hydrogels may repair themselves after breaking, extending their useful life and reducing the risk of wound infections (Li et al. 2023). To study the self-healing behavior of S2 and S5 hydrogels, each hydrogel disc was cut into two halves and brought into physical contact (Fig. 3). Consequently, the author lifted the hydrogel after healing, and it can be seen restoring the mechanical properties of the hydrogel after self-healing. Also, as shown in Fig. 3, the hydrogels were dyed individually with methylene blue and methyl red into blue and red and molded in a rectangular-shaped mold. After cutting the hydrogel, the hydrogel was simulated as destroyed, and the healing status was observed as the two hydrogels healed together. However, by bringing the two halves into contact, hydrogen bonds between hydroxyl and amino groups in the hydrogel and amide bond between grafted alginate and drug-loaded functional groups reformed, recovering the hydrogel.
Rheology studies
The rheological properties of the hydrogel are essential factors in determining the mechanical properties of the hydrogels. Consequently, we have measured the viscosity and Torque of the hydrogel loaded with 9-AA in a range of 1–100 s−1 of shear rate (Fig. 4). Accordingly, it showed a shear-thinning behaviour, non-Newtonian behaviour as viscosity decreases under shear strain, and a high drop of its viscosity was observed. Concurrently, the Torque, the material's resistance to the shearing action, was increased by increasing the shear rate (Belalia and Djelali 2014).
Adhesive property of hydrogels
The hydrogels were checked for their adhesion ability to various substrates. Figure 5 shows the strong adhesive ability of the hydrogels, S2 and S3, on stainless steel, glass, rubber, and plastic. The adhesion ability of the hydrogels to adhere to the substrates surfaces is due to the strong hydrogen bonding between the substrate surface (plastic, metal, glass, or rubber) and the hydrogel's different functional groups. The free hydroxyl and amino groups dispersed in the hydrogel could react with amine or thiol-containing substrates via Michael-type addition reactions or aryl–aryl coupling (Lee et al. 2006). Also, many carbonyl groups in hydrogel chains can contribute to adhesiveness through amide bonds with the amino group.
Electrical conductivity
The typical frequency dependence at room temperature on the conductivity function σ′ and iσ′′ for the prepared hydrogel was presented in Fig. 6. From the Figure, at 100 kHz frequencies, the transportation of the charge is the response of conductivity and dielectric functions. Also, from Figure, it is noticed that at ~ 106 Hz, the complex conductivity (σ″) reduced to a minimum value followed by sharp increase of the real permittivity (ε′), which is the beginning of the electrode polarization development. At the critical frequency (νc), the conductivity rises parallel with the decrease in real permittivity. This phenomenon is characteristic of the complete build-up of the electrochemical double layer, or what is known as electrode polarization. Decreasing frequency showed an upward bending-like behavior at 10 Hz in imaginary and real parts of the complex conductivity, which is the final blocking of a second charge-transport mechanism.
Cytocompatibility study by MTT assay
Cytocompatibility study by MTT assay
MTT detection of capability is considered a reliable, sensitive, and quantitative cell viability assay (Ogbole et al. 2017). In this assay, the cellular mitochondrial dehydrogenase reduction capacity of the yellow water-soluble substrate 3-(4,5-dimethylthiazol-2yl)-2,5-diphenyl tetrazolium bromide (MTT) taken by the living cells. The formazan of the insoluble dark blue/purple formazan product is directly proportional to the number of living cells. MTT assay is used to determine the cytotoxic effect of both the hydrogel with and without the drugs, 9-AA, and KS, the results are shown in Fig. 7.
Cytocombitability of the hydrogels using acridine orange staining
Further investigation for cytocompatibility was done using acridine orange dye to determine the viability of cells after treatment, and the results are shown in Fig. 8. When the hydrogel without the drugs (10 mg/mL) was incubated with the cells in 96 well plates, the MTT assay revealed a reduction of 70% in the viable cells, which can be attributed to the hydrogel's ability to absorb the media from the well leading to insufficient nutrient which may contribute to increased cell death. Surprisingly, the same hydrogel at the same concentration displayed a negligible cytotoxic effect on the cells when live/dead cells assay was applied, Fig. 8. Meanwhile, 9-AA exhibited a powerful cytotoxic effect in a dose-dependent manner. It is almost toxic at 0.25 and 0.5 µg/mL, as shown in Fig. 8a, b. Also, this contributes to the determined IC50 of 9-AA after MTT assay 0.3 µg/mL. Instead, KS at 0.5 µg/mL was safe and supported cell growth and proliferation.
The staining of live cells with acridine orange revealed the following observations.
-
Hydrogels encapsulating 9-AA with three different loading percents 92.5, 88.6, and 86.9%, displayed minimal toxicity against the HFB-4 cells even with increasing the hydrogel mass (10, 20, and 30 mg), suggesting efficient encapsulation of the drugs in the hydrogel and its sustained release at pH 7.4.
-
The cytocompatibility for the hydrogel loaded with 9-AA was comparable to the hydrogel without the drugs, as indicated in figures a (I-III), b (I-III), c (I-III).
-
Similarly, the hydrogel loaded with 89.3% KS loading was cytocompatible at all treatments, with a notable increase in the cell population with increasing hydrogel masses, Fig. 7d (I–III) (Gu et al. 2022).
-
9-AA toxic effect was detected on cells when 0.25 and 0.5 µg/mL were incubated with monolayers HFB-4 cells. o the other hand, the free kanamycin at 0.5 µg/mL concentration, was considered safe on cell monolayers. The cytotoxicity of 9-AA could be explained by the reported ability of 9-AA to inhibit the activity of topoisomerase enzymes and other regulatory proteins such as PI3K/AKT/mTOR, p53, and TNF, that regulate the proliferation and migration (Baguley et al. 2003; El-Sayed et al. 2022b; Mangueira et al. 2022).
In vitro wound healing assessment
The efficacy of the S50K and S50A hydrogels in mediating the normal skin cells to heal the scratch was assessed in vitro using normal human skin fibroblast cells (HFB-4 cells). Following the simple cell migration assay (Bektas et al. 2020), a linear thin scratch (wound) in a confluent cell monolayer was created (Fig. 9). Afterward, the images of the cells merging the gap at different time intervals showed the effectiveness of the active compounds in curing the scratch (Fig. 10). The scratch examination after treatment revealed that S50K was the most potent in accelerating the wound healing process by decreasing the scratch gap from both sides by 26.3%. On the other hand, the ability of S50A to heal the induced scratch was neglectable (the gap between the two scratch sides was reduced by 2.5%). However, treating the scratch with a mixture of hydrogels led to a notable gab-closing ability but still less than S50K. Overall, the results reflected a remarkable impact of S50K in inducing the scratch closing within 6 h compared to S50A.
Conclusion
In summary, a self-healing hydrogel with conductive and adhesive properties was prepared by grafting alginate with poly(β-carboxyethyl acrylate-co-acrylamide) to have a carboxylic and carbonyl bonds, which can form a dynamic bond. In addition, 9-Aminoacridine and kanamycin sulfate were added to improve the antibacterial and anti-infection activities. As a result, the prepared hydrogels displayed electrical conductivity and adhesive with non-Newtonian fluid and could heal after damage. In addition, the hydrogels had antimicrobial and cytotoxicity properties, allowing them to have potential wound-healing applications. Finally, the in vitro wound-healing assay revealed the promising ability of the hydrogel-encapsulating kanamycin to induce scratch-healing within six hours by 26.3%.
Availability of data and materials
The data sets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
Autumn K, Sitti M, Liang YA, Peattie AM, Hansen WR, Sponberg S, Kenny TW, Fearing R, Israelachvili JN, Full RJ (2002) Evidence for van der Waals adhesion in gecko setae. Proc Natl Acad Sci 99:12252–12256
Baguley BC, Wakelin LP, Jacintho JD, Kovacic P (2003) Mechanisms of action of DNA intercalating acridine-based drugs: how important are contributions from electron transfer and oxidative stress? Curr Med Chem 10:2643–2649
Bektas N, Şenel B, Yenilmez E, Özatik O, Arslan R (2020) Evaluation of wound healing effect of chitosan-based gel formulation containing vitexin. Saud Pharm J 28:87–94
Belalia F, Djelali N-E (2014) Rheological properties of sodium alginate solutions. Rev Roum Chim 59:135–145
Cao J, Xiao L, Shi X (2019) Injectable drug-loaded polysaccharide hybrid hydrogels for hemostasis. RSC Adv 9:36858–36866
Chen T, Chen Y, Rehman H, Chen Z, Yang Z, Wang M, Li H, Liu H (2018) Ultratough, self-healing, and tissue-adhesive hydrogel for wound dressing. ACS Appl Mater Interfaces 10:33523–33531
Du X, Hou Y, Wu L, Li S, Yu A, Kong D, Wang L, Niu G (2020) An anti-infective hydrogel adhesive with non-swelling and robust mechanical properties for sutureless wound closure. J Mater Chem B 8:5682–5693
El-Sayed NS, Moussa MA, Kamel S, Turky G (2019) Development of electrical conducting nanocomposite based on carboxymethyl cellulose hydrogel/silver nanoparticles@ polypyrrole. Synth Met 250:104–114
El-Sayed NS, Al Kiey SA, Darwish A, Turky G, Kamel S (2022a) High performance hydrogel electrodes based on sodium alginate-g-poly (AM-c o-ECA-co-AMPS for supercapacitor application. Int J Biol Macromol 218:420–430
El-Sayed NS, Hashem AH, Kamel S (2022b) Preparation and characterization of Gum Arabic Schiff’s bases based on 9-aminoacridine with in vitro evaluation of their antimicrobial and antitumor potentiality. Carbohyd Polym 277:118823
El-Sayed NS, Salama A, Guarino V (2022c) Coupling of 3-aminopropyl sulfonic acid to cellulose nanofibers for efficient removal of cationic dyes. Materials 15:6964
El-Sayed NS, Hashem AH, Khattab TA, Kamel S (2023) New antibacterial hydrogels based on sodium alginate. Int J Biol Macromol 248:125872
Froelich A, Jakubowska E, Wojtyłko M, Jadach B, Gackowski M, Gadziński P, Napierała O, Ravliv Y, Osmałek T (2023) Alginate-based materials loaded with nanoparticles in wound healing. Pharmaceutics 15:1142
Gu J, Zhang S, Xia X, Zhang X, Fan B, Zhou J, Zhu H, Wang W, Qi X, Li L (2022) An edible kanamycin sulfate cross-linked cellulose active against multiple pathogenic bacteria. Int J Biol Macromol 194:435–444
Jafarigol E, Salehi MB, Mortaheb HR (2020) Preparation and assessment of electro-conductive poly (acrylamide-co-acrylic acid) carboxymethyl cellulose/reduced graphene oxide hydrogel with high viscoelasticity. Chem Eng Res Des 162:74–84
Kamel S, Dacrory S, Hesemann P, Bettache N, Ali LM, Postel L, Akl EM, El-Sakhawy M (2023) wound dressings based on sodium alginate-polyvinyl alcohol–moringa oleifera extracts. Pharmaceutics 15:1270
Kamoun EA, Kenawy E-RS, Chen X (2017) A review on polymeric hydrogel membranes for wound dressing applications: PVA-based hydrogel dressings. J Adv Res 8:217–233
Khattab TA, Kamel S (2022) Advances in polysaccharide-based hydrogels: Self-healing and electrical conductivity. J Mol Liq 352:118712
Lazow SP, Labuz DF, Freedman BR, Rock A, Zurakowski D, Mooney DJ, Fauza DO (2021) A novel two-component, expandable bioadhesive for exposed defect coverage: applicability to prenatal procedures. J Pediatr Surg 56:165–169
Lee H, Scherer NF, Messersmith PB (2006) Single-molecule mechanics of mussel adhesion. Proc Natl Acad Sci 103:12999–13003
Li M, Liang Y, Liang Y, Pan G, Guo B (2022a) Injectable stretchable self-healing dual dynamic network hydrogel as adhesive anti-oxidant wound dressing for photothermal clearance of bacteria and promoting wound healing of MRSA infected motion wounds. Chem Eng J 427:132039
Li M, Pan G, Zhang H, Guo B (2022b) Hydrogel adhesives for generalized wound treatment: design and applications. J Polym Sci 60:1328–1359
Li R, Qi Q, Wang C, Hou G, Li C (2023) Self-healing hydrogels fabricated by introducing antibacterial long-chain alkyl quaternary ammonium salt into marine-derived polysaccharides for wound healing. Polymers 15:1467
Lu W, Bao D, Ta F, Liu D, Zhang D, Zhang Z, Fan Z (2020) Multifunctional alginate hydrogel protects and heals skin defects in complex clinical situations. ACS Omega 5:17152–17159
Mangueira V, de Sousa T, Batista T, de Abrantes R, Moura A, Ferreira R, de Almeida R, Braga R, Leite F, Medeiros K (2022) A 9-aminoacridine derivative induces growth inhibition of Ehrlich ascites carcinoma cells and antinociceptive effect in mice. Front Pharmacol 13:963736
Nishiguchi A, Kurihara Y, Taguchi T (2020) Hemostatic, tissue-adhesive colloidal wound dressing functionalized by UV irradiation. ACS Appl Bio Mater 3:1705–1711
Ogbole OO, Segun PA, Adeniji AJ (2017) In vitro cytotoxic activity of medicinal plants from Nigeria ethnomedicine on Rhabdomyosarcoma cancer cell line and HPLC analysis of active extracts. BMC Complement Altern Med 17:1–10
Ono K, Saito Y, Yura H, Ishikawa K, Kurita A, Akaike T, Ishihara M (2000) Photocrosslinkable chitosan as a biological adhesive. J Biomed Mater Res Off J Soc Biomater Jpn Soc Biomater 49:289–295
Qu J, Zhao X, Liang Y, Zhang T, Ma PX, Guo B (2018) Antibacterial adhesive injectable hydrogels with rapid self-healing, extensibility and compressibility as wound dressing for joints skin wound healing. Biomaterials 183:185–199
Sood A, Granick MS, Tomaselli NL (2014) Wound dressings and comparative effectiveness data. Adv Wound Care 3:511–529
Thomé MP, Filippi-Chiela EC, Villodre ES, Migliavaca CB, Onzi GR, Felipe KB, Lenz G (2016) Ratiometric analysis of Acridine Orange staining in the study of acidic organelles and autophagy. J Cell Sci 129:4622–4632
Vk Nelson, Sahoo NK, Sahu M, Sudhan HH, Pullaiah CP, Muralikrishna KS (2020) In vitro anticancer activity of Eclipta alba whole plant extract on colon cancer cell HCT-116. BMC complement med ther 20:1–8
Wang R, Zhu J, Jiang G, Sun Y, Ruan L, Li P, Cui H (2020) Forward wound closure with regenerated silk fibroin and polylysine-modified chitosan composite bioadhesives as dressings. ACS Appl Bio Mater 3:7941–7951
Wu G, Jin K, Liu L, Zhang H (2020) A rapid self-healing hydrogel based on PVA and sodium alginate with conductive and cold-resistant properties. Soft Matter 16:3319–3324
Zhang X, Wang D, Liu H, Yue L, Bai Y, He J (2020) A nucleobase-inspired super adhesive hydrogel with desirable mechanical, tough and fatigue resistant properties based on cytosine and ε-caprolactone. Eur Polymer J 133:109741
Zhang Y, Chen Q, Dai Z, Dai Y, Xia F, Zhang X (2021) Nanocomposite adhesive hydrogels: from design to application. J Mater Chem B 9:585–593
Zhao X, Liang Y, Huang Y, He J, Han Y, Guo B (2020) Physical double-network hydrogel adhesives with rapid shape adaptability, fast self-healing, antioxidant and NIR/pH stimulus-responsiveness for multidrug-resistant bacterial infection and removable wound dressing. Adv Func Mater 30:1910748
Zheng D, Xu X, Zhu J, Bai B, Wang Q, Shi W, Li J (2023) Humidity capture and solar-driven water collection behaviors of alginate-g-PNIPAm-based hydrogel. J Environ Chem Eng 11:109247
Acknowledgements
The authors acknowledge the National Research Center (NRC), Egypt, for financial support of the current work.
Funding
Open access funding provided by The Science, Technology & Innovation Funding Authority (STDF) in cooperation with The Egyptian Knowledge Bank (EKB).
Author information
Authors and Affiliations
Contributions
NSES contributed to conceptualization, performed all chemical synthesis and materials characterization, writing and reviewing the manuscript. NMH contributed to cytocompatibility study, wound healing assay, writing and reviewing the manuscript. SK contributed to conceptualization, validation, and reviewing the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethics approval
Not applicable.
Consent to participate
Not applicable.
Consent for publication
The authors approve the publication of the manuscript.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
El‑Sayed, N.S., Helmy, N.M. & Kamel, S. Dual-adhesive and self-healing alginate-based hydrogel for wound healing. Chem. Pap. 78, 1021–1031 (2024). https://doi.org/10.1007/s11696-023-03140-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11696-023-03140-4