Abstract
Recently, iron(II) oxalate has experienced a renewed interest due to their newly found application in lithium-ion batteries. Lithium is expected to be embedded between the oxalate sheets, dramatically increasing the need to understand the oxalate structure. Despite being known for decades, the discrepancies still exist regarding the anhydrous iron(II) oxalate. In this work, we explore the dehydration process of both α-FeC2O4·2H2O and β-FeC2O4·2H2O polymorphs at different heating rates and calcination temperatures by X-ray powder diffraction, Mössbauer spectroscopy and scanning electron microscopy. After dehydration, iron(II) oxalates formed two polymorphs with different XRD patterns: α-FeC2O4 with sharp and narrow diffraction lines and β-FeC2O4 with very broadened lines, which were attributed to the monoclinic structure with space group P21/n.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
In the last decades, iron(II) oxalate was used on numerous occasions as a precursor for the synthesis of various iron oxides and carbides. For example, the thermal decomposition of iron(II) oxalate in an air atmosphere is well known to produce α-Fe2O3 (hematite) (Hermanek et al. 2007; Zhou et al. 2008). Reactions in inert atmospheres are more complex; in a nitrogen atmosphere, the oxalate decomposes to Fe3O4 (magnetite) and α-Fe via an unstable phase of FeO (wüstite) (Boyanov et al. 1985; Brown and Bevan 1966); in the atmosphere of conversion gases, the phases of Fe3O4 and Fe3C (cementite) have been found among the decomposition products (Hermanek et al. 2006).
More recently, the iron(II) oxalate was also suggested as a novel material for lithium-ion batteries, due to their good reversible capacity, cycling performance, environmental friendliness and low cost, which has renewed the scientific interest in these metalorganic materials (Zhang et al. 2020; He et al. 2021; Li et al. 2021; Song et al. 2023). It has been shown that the crystal structure and morphology play an important role in their electrochemical performance and the stability of their diffusion channels for Li+ ions.
Metal oxalates are frequently encountered as hydrates, where water molecules have a crucial role in the crystal structure formation. The iron(II) oxalate dihydrate molecules form long chains in which iron cations and oxalate anions alternate periodically. The two water molecules are attached to each iron cation, completing the bonding octahedron of the iron atom.
The individual chains are then linked via hydrogen bonding (see Fig. S1). The mutual bonding of these oxalate chains gives rise to the two structurally different polymorphs: α- and β-polymorph.
The α-FeC2O4·2H2O has a monoclinic crystal structure, space group C2/c, with lattice parameters: a = 12.06 Å, b = 5.57 Å, c = 9.76 Å, and β = 124.3°. The β-polymorph crystalizes in an orthorhombic crystal structure, space group Cccm, with a = 12.26 Å, b = 5.57 Å and c = 15.48 Å (Naumov et al. 1996). Both polymorphs can be prepared separately by a precipitation method from ferrous salt and oxalic acid (Angermann and Töpfer 2008).
It is known that iron(II) oxalate dihydrate starts to lose its crystal water within the temperature range of 160‒210 °C, regardless of reaction atmosphere and type of the polymorph (Mohamed et al. 2005; Angermann and Töpfer 2008). In oxygen-rich reaction atmospheres, dehydration process is followed very rapidly by a thermally induced decomposition of the oxalate to Fe2O3. Generally, when observed with DSC (differential scanning calorimetry), these two events, i.e., dehydration and decomposition, overlap (Mohamed et al. 2005). However, the anhydrous form can be prepared in inert atmosphere or vacuum, where the anhydrous iron(II) oxalate is stable up to 320 °C (Katada and Ogimoto 2000; Macklen 1967).
However, there is very little information in the literature on the crystal structure of anhydrous oxalates, particularly FeC2O4. Kondrashev et al. reported that most of the divalent metal anhydrous oxalates MC2O4 are isostructural and can be divided to disordered α- and ordered β-phase (Kondrashev et al. 1985). For the β-phase of iron(II) oxalate, they proposed a monoclinic crystal structure with P21/n space group and lattice parameters a = 5.76 Å, b = 5.07 Å, c = 5.50 Å, and β = 113.45°. Nonetheless, Kondrashev et al. also noted that additional diffraction peaks could be observed in the case of anhydrous iron(II) oxalate, which they suggested might indicate the presence of another phase. These additional sharp diffraction peaks are also included in the PDF-14-0807 card, which is traditionally used to identify anhydrous iron(II) oxalate in the diffraction patterns and dates back to the work Brown and Bevan (Brown and Bevan 1966). In this case, the associated phase is usually referred to simply as “FeC2O4”. However, many acquired iron(II) oxalate patterns in the literature do not contain these sharp lines (Zhang et al. 2020; Ogasawara and Koga 2014; Koga and Sato 2011). On the contrary, they match the patterns of other transition metal oxalates as stated originally by Kondrashev et al. The absence of these lines is usually explained by an increased number of crystal defects or poor crystallinity.
More recently, Zhang et al. used the term “α-FeC2O4” to refer to the phase that contained all lines present in the PDF-14-0807 card (Zhang et al. 2019). On the other hand, they used the term “β-FeC2O4” to describe the disordered structure that exhibited a diffraction pattern similar to that of anhydrous ZnC2O4 or CuC2O4 (Christensen et al. 2014; Kolezynski and Malacki 2009). Nonetheless, they were not able to identify the crystal structure of this α-FeC2O4. Importantly, according to Zhang et al. these two forms exhibit different electrochemical properties (Zhang et al. 2019).
Although the polymorphism of the anhydrous oxalates was of little importance in the past, mainly because it did not affect the thermal decomposition reactions of oxalates, the importance of oxalate polymorphism and the means to control it have grown rapidly in light of recently found application potential of the anhydrous iron(II) oxalate in lithium-ion batteries (Zhang et al. 2020).
In this work, we investigate the dehydration of both iron(II) oxalate dihydrate polymorphs in order to establish the experimental conditions for the selective preparation of different anhydrous forms of iron(II) oxalate found in the literature. Although we were able to prepare only one polymorph of anhydrous iron(II) oxalate in pure form, we bring new information on the effects of the heating rate and the terminal temperature on the process of dehydration of iron(II) oxalate dihydrate. Both hydrated and anhydrous phases were studied with X-ray powder diffraction, Mössbauer spectroscopy and scanning electron microscopy.
The controlled synthesis of different anhydrous polymorphs in a pure form is necessary for future identification of their crystal structure, which is a key step for understanding the electrochemical properties of these materials.
Experimental
Synthesis of iron(II) oxalate dihydrate
Samples of iron(II) oxalate dihydrate were prepared by a precipitation method (Angermann and Töpfer 2008; Lisníková et al. 2022; Zhang et al. 2020).
Briefly, β-FeC2O4·2H2O was prepared by dissolving 15.67 mmol of iron(II) chloride tetrahydrate (99%, Penta Ltd.) in 50 ml of deionized water. This solution was drop-wise added into a solution of 23.5 mmol oxalic acid (99.8%, Lach-Ner Ltd.) in 50 ml of deionized water with a constant stirring. All reagents were used without further purification. This mixing process took approximately 1.5 h. The precipitate was afterward filtered, washed with deionized water and isopropyl alcohol and then left to dry in vacuum.
Similarly, α-FeC2O4·2H2O was prepared by the precipitation method, but in this case the solutions were kept at 90 °C during both mixing and prolonged stirring (another 90 min). The final solution was let to cool down and stirred for another 16 h at room temperature. Afterward, the precipitate was filtered, washed with deionized water and left in dry air atmosphere.
Preparation of anhydrous iron(II) oxalate
Anhydrous iron(II) oxalate was prepared by calcination of iron(II) oxalate dihydrate samples in dynamic nitrogen atmosphere in a laboratory furnace (Carbolite Gero HTR1100). In the first series, the samples were heated to 200 °C with different heating rates (1, 2, 10 and 20 °C/min) and calcinated for 2 h (samples α-200-1, α-200-2, α-200-10, α-200-20 and β-200-1, β-200-2, β-200-10, β-200-20). In the second series, heating rate was kept constant, 10 °C/min, while the terminal temperature was changed from 200 °C to 250 °C and to 300 °C (samples α-200-10, α-250-10, α-300-10 and β-200-10, β-250-10, β-300-10). Both α-FeC2O4·2H2O and β-FeC2O4·2H2O samples were calcinated simultaneously to ensure the same heating conditions.
Experimental equipment
X-ray diffraction (XRD) patterns of all samples were obtained by Bruker D8 ADVANCE diffractometer with Bragg–Brentano geometry employing CoKα radiation over the 2θ range of 10–100° with a step of 0.02°. Divergence slit 0.6 mm and 2.5° axial Soller slits were inserted into the primary beam path, whereas Fe Kβ filter and 2.5° axial Soller slits were added to the secondary beam path. The measured XRD patterns were analyzed by Rietveld analysis in MAUD program (Lutterotti et al. 2010). In the case of high-temperature in situ XRD experiments 30–900–30 °C, the samples of commercially available iron(II) oxalate (Sigma-Aldrich) were put in the Anton Paar XRK900 high-temperature chamber. After reaching the set temperature, the heating was stopped and XRD pattern was recorded. The measurements were taken at 10 °C temperature steps, and each took approximately 20 min.
The morphology of prepared oxalate samples was studied using Tescan VEGA 3 LMU scanning electron microscope (SEM). The accelerating voltage was set to 30 kV. Samples were coated with 20 nm of chromium to minimize the sample charging using Quorum QT 150T ES sputtering device.
Lastly, the samples of iron oxalates were inspected by Mössbauer spectroscopy. Transmission 57Fe Mössbauer spectra were collected at room temperature using OLTWINS dual spectrometer developed at Palacký University Olomouc, Czech Republic (Stejskal et al. 2023). The dual spectrometer utilizes a universal drive unit capable of carrying heavy loads (Novák et al. 2022) and unique autotuning procedure (Procházka et al. 2020). A radiation source 57Co embedded in rhodium matrix was used, and the spectra were processed using MossWinn 4.0 software (Klencsár et al. 1996). All the isomer shifts were given with respect to metallic α-Fe at room temperature.
Results and discussion
Characterization of iron(II) oxalate dihydrate
Prepared samples of iron(II) oxalate dihydrate were firstly examined by X-ray powder diffraction. The obtained patterns revealed that precipitation and aging at 90 °C led to the monoclinic α-iron(II) oxalate dihydrate (PDF 023-0293) shown in Fig. 1a. For the Rietveld analysis (Fig. S2), the crystal model COD-7218522 from Crystallography Open Database was used (Graulis et al. 2009; Tominaka and Cheetham 2014). This resulted in lattice parameters: a = 12.06 Å, b = 5.56 Å, c = 9.95 Å and β = 128.5°, which are in good agreement with α-iron(II) oxalate dihydrate parameters reported by other authors (Echigo and Kimata 2008; Tominaka and Cheetham 2014; Angermann and Töpfer 2008).
The XRD diffraction pattern of the sample prepared at a laboratory temperature is shown in Fig. 1b. All diffraction peaks could be indexed in the orthorhombic structure (space group Cccm) of β-iron(II) oxalate dihydrate (PDF 022-0635). For Rietveld analysis, the crystal model ICSD-109902 from Inorganic Crystal Structure Database was used (Fig. S3) (Deyrieux and Peneloux 1969), which led to the lattice parameters of: a = 12.49 Å, b = 5.56 Å and c = 15.46 Å. The presence of additional diffraction lines in the model that are not in the measured pattern can be solved by incorporating a model with an ordered/disordered crystal structure with shifted crystal lattice in the plane of the oxalate chains propagation (Christensen et al. 2014). The model is shown in Fig. S4 and resulted in lattice parameters: a = 12.48 Å; b = 5.56 Å; and c = 15.47 Å. The reflections corresponding to planes that lie parallel to a-axis (e.g. (400), (602)) of the crystal structure are unusually wide compared to other diffraction peaks. This suggests that the used crystal model of β-iron(II) oxalate dihydrate may need further improvements, but these are beyond the scope of this article. Both samples were prepared as a pure fraction.
The two polymorphs also differed in their particle size. The α-iron(II) oxalate dihydrate had significantly larger and separated particles with size in the range 4–20 μm as demonstrated in Fig. 2a. On the contrary, the sample of β-iron(II) oxalate dihydrate contained small, agglomerated rod-shaped particles with a particle size in order of 2–6 μm shown in Fig. 2b.
Both iron(II) oxalate dihydrate samples were further examined with Mössbauer spectroscopy. The Mössbauer spectra in Fig. 3 exhibit doublets, which are typical for iron-bearing oxalates (Aramu et al. 1977; Hermanek et al. 2006; Smrčka et al. 2016; Kopp et al. 2019). Considering the uncertainty of the measurement, the hyperfine parameters for both polymorphs were almost identical, which indicates that the FeO6 bonding octahedrons are unchanged despite the different crystal structure, i.e., the different mutual linkage of the oxalate chains.
The values for α- and β-iron(II) oxalate dihydrate samples were following: isomer shift (1.20 ± 0.01) and (1.19 ± 0.01) mm/s, respectively, quadrupole splitting of (1.72 ± 0.01) mm/s and (1.70 ± 0.01) mm/s, respectively. As shown by Aramu et al., Mössbauer spectroscopy cannot distinguish between the two hydrated polymorphs (Aramu et al. 1977).
Characterization of anhydrous iron(II) oxalate
The calcination temperature of 200 °C for dehydrating the samples was selected based on high-temperature in situ XRD experiments conducted in air and nitrogen atmospheres. These experiments were performed over a wide temperature range, from 30 °C up to 900 °C and then back to 30 °C. The results of these experiments can be found in Figures S5 and S6. They show that in air the FeC2O4·2H2O dehydrated and decomposed simultaneously, while in nitrogen atmosphere the dehydration started at approximately 180 °C and was followed by the decomposition only after reaching 300 °C.
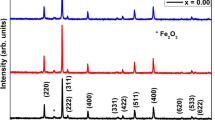
The dehydration of iron(II) oxalate dihydrate samples induced the structural changes, which resulted in a creation of a new set of diffraction lines in the XRD patterns. Most of the patterns exhibited a set of very narrow lines as well as a few very broadened lines. The position of these observed lines agreed well with the PDF-14-0807 diffraction lines. The mutual intensity between the narrow and broaden lines was found to variate depending on the conditions of dehydration, e.g., the rate of increasing temperature. The data in Fig. 4 show the effect of the used precursor and of the selected heating rate. Both series of samples, i.e., from α-200-1 to α-200-20 and from β-200-1 to β-200-20, always contained both narrow and broadened peaks. Moreover, the relative intensity of the narrow peaks grew with the increasing rate of heating. Almost no narrow peaks were found in β-200-1, i.e., the sample calcined with the slowest heating rate. The positions of the broadened peaks (most intensive of them are marked with β symbol in Fig. 4b) in β-200-1 pattern corresponded well to those of the disordered structures of other transition metal oxalate (Cu, Zn, etc.) described by Kondrashev et al. Similarly to Zhang et al., we denoted the phase with broadened lines as β-FeC2O4 (Zhang et al. 2019). The set of narrow lines (the most intensive of them are marked with α symbol in Fig. 4a) signaled the presence of another phase, which we denoted as α-FeC2O4. Using this classification, we state that most of the samples contained a mixture of α- and β-anhydrous iron(II) oxalate polymorphs with small traces of hydrated precursors as shown in Fig. 4. The almost pure phase of β-iron(II) oxalate was prepared by slow calcination at heating rate 1 °C/min in nitrogen atmosphere by dehydrating β-iron(II) oxalate dihydrate precursor.
Figure 5 shows the influence of the terminal temperature on the dehydration process. In the case of both precursors, we observe no noticeable effect of the maximum temperature value on the resultant XRD patterns. At higher temperatures, the traces of precursors disappeared; however, there was only a slight change in the α-anhydrous iron(II) oxalate diffraction line intensities.
X-ray powder diffraction patterns of α- and β-anhydrous iron(II) oxalate samples after dehydration process in nitrogen atmosphere with a heating rate of 10 °C/min and different terminal temperatures 200 °C, 250 °C and 300 °C: a diffraction peaks of samples α-(200–300)-10 and b samples β-(200–300)-10
The morphology of oxalate samples after the dehydration process remained the same in comparison with their iron(II) oxalate dihydrate counterparts. This can be seen in Fig. 6.
Sample α-200-20 containing a combination of α- and β-iron(II) oxalate had large and separated particles (Fig. 6a). On the contrary, sample β-200-1 containing almost pure fraction of β-iron(II) oxalate contained small agglomerated rod-shaped particles (Fig. 6b).
In order to support our assumptions that the narrow and broadened peaks belong to different anhydrous oxalate phase, we examined the samples with Mössbauer spectroscopy. In Fig. 7a, the spectrum of sample β-200-1, there is only one doublet visible with isomer shift of (1.19 ± 0.01) mm/s and quadrupole splitting of (1.65 ± 0.01) mm/s. This doublet could be associated with anhydrous β-iron(II) oxalate. On the other hand, in Fig. 7b, in the spectrum of sample α-200-20, there are two doublets with similar isomer shift, (1.20 ± 0.01) and (1.22 ± 0.01) mm/s, but significantly different quadrupole splitting of (1.65 ± 0.01) and (2.23 ± 0.01) mm/s. The first doublet could be referred to β-iron(II) oxalate as it is identical to the one in the spectrum of sample β-200-1. The other doublet we ascribed to the α-iron(II) oxalate. This increased quadrupole splitting was also reported before, and it is in good agreement with the previously measured data (Hermanek et al. 2006). The difference in the quadrupole splitting suggests two different environments of iron cations, i.e., different deformations of the FeO6 bonding octahedron caused by differences in the mutual linking of the oxalate chains. Contrary to the hydrated forms, the anhydrous polymorphs of iron(II) oxalate can be distinguished by Mössbauer spectroscopy.
Recently, Christensen et al. (2014) proposed order/disorder model for CuC2O4·nH2O with a space group P21/n (Christensen et al. 2014). The copper(II) hydrate forms an exception among other transition metal oxalates where the water is not part of the lattice, but it is adsorbed in “zeolite-like” manner. Therefore, it was suggested that the CuO6 octahedron is formed by forming bonds between the Cu ions and the atoms of nearby chains. Due to the absence of official structural models for anhydrous iron(II) oxalates and by assuming the similar bonding behavior to CuC2O4, we propose a structural model for β-FeC2O4. We used space group P21/n and crystal parameters: a = 5.76 Å, b = 5.07 Å, c = 5.50 Å, and β = 113.45° originally suggested by Kondrashev et al. (1985) and stacking disorder formulated by Christensen et al. The Rietveld refinement fit is shown in Fig. 8. The refined crystal parameters of a = 5.75 Å, b = 5.32 Å, c = 5.43 Å and β = 116.8° were found. Atomic positions are listed in Table 1. The good agreement between the data and the fit suggests that our assumptions concerning the structure are probably correct. However, the structure behind the narrow lines, i.e., α-FeC2O4, still remains unclear. We were not able to propose a model for this anhydrous polymorph of iron(II) oxalate due to the lack of available information, and also because we were not able to prepare this polymorph in pure form under the studied conditions. In this, the current work is still a work in progress and future efforts will be needed to isolate crystalline anhydrous α-FeC2O4 and to reveal its structure with single-crystal XRD.
XRD pattern of the calcinated sample of β-200-1 in comparison with PDF 14–0807 pattern from database. The unit cell of anhydrous β-iron(II) oxalate, space group P21/n, was modeled in VESTA software (Momma and Izumi 2011)
Conclusions
We successfully prepared and analyzed two known polymorphs of α- and β-iron(II) oxalate dihydrate. Although some discrepancies concerning the anhydrous iron(II) oxalate were addressed, further extended research will be required. Based on our investigation, it appears that similarly to hydrated form there exist at least two dehydrated polymorphs that we denominate α- and β-iron(II) oxalate. Their formation was found to be dependent on dehydration conditions.
After calcination in nitrogen, most XRD patterns exhibited very narrow peaks, which we ascribed to anhydrous α-iron(II) oxalate, and broadened peaks we associated with anhydrous β-iron(II) oxalate. The mutual intensity of diffraction lines depended on the selected heating rate and on the selected precursor (either α- or β-iron(II) oxalate dihydrate). The terminal temperature of dehydration appeared to have minimal effect as long as it did not exceed the oxalate thermal stability of 300 °C. Nonetheless, it should be noted that other factors such as particle size or the rate of the gas flow during the dehydration cannot be excluded as they were not tested. In the case of α-iron(II) oxalate dihydrate, the patterns of dehydrated samples always contained both the narrow peaks of anhydrous α-iron(II) oxalate and the broadened peaks of anhydrous β-iron(II) oxalate. On the other hand, in the case of β-iron(II) oxalate dihydrate, the almost pure anhydrous β-iron(II) oxalate could be prepared at the slowest heating rate of 1 °C/min.
The formation and existence of two different anhydrous polymorphs are supported with results of Mössbauer spectroscopy, where the spectra displayed two different doublets suggesting two differently deformed bonding FeO6 octahedrons. The crystal structure of anhydrous β-iron(II) oxalate forms a monoclinic cell (P21/n) that is isostructural with other anhydrous transition metal oxalates, e.g., ZnC2O4 or CuC2O4. The observed β-iron(II) oxalate pattern could be reasonably fitted using the proposed model with an incorporated stacking fault. The introduction of stacking faults suppressed the intensity of certain reflections. On the other hand, the structure of anhydrous α-iron(II) oxalate appears to be unique and not isostructural with other known anhydrous oxalates. The symmetry and lattice parameters remain unknown. Based on the current scientific interest, the oxalate crystal structures and their low-temperature transformations certainly deserve further examination and research.
References
Angermann A, Töpfer J (2008) Synthesis of magnetite nanoparticles by thermal decomposition of ferrous oxalate dihydrate. J Mater Sci 43(15):5123–5130. https://doi.org/10.1007/s10853-008-2738-3
Aramu F, Maxia V, Muntoni C (1977) Mössbauer spectroscopy of polymorphous iron oxalate. Hyperfine Interact 5(1):399–402. https://doi.org/10.1007/BF01021710
Boyanov B, Khadzhiev D, Vasilev V (1985) Study of thermal decomposition of FeC204·2H20. Thermochim Acta 93(September):89–92. https://doi.org/10.1016/0040-6031(85)85023-1
Brown RA, Bevan SC (1966) The thermal decomposition of ferrous oxalate dihydrate. J Inorg Nucl Chem 28:387–391. https://doi.org/10.1016/0022-1902(66)80316-0
Christensen AN, Lebech B, Andersen NH, Grivel JC (2014) The crystal structure of paramagnetic copper(II) oxalate (CuC2O4): formation and thermal decomposition of randomly stacked anisotropic nano-sized crystallites. Dalton Trans 43(44):16754–16768. https://doi.org/10.1039/c4dt01689k
Echigo T, Kimata M (2008) Single-crystal X-ray diffraction and spectroscopic studies on humboldtine and lindbergite: weak Jahn-Teller effect of Fe2+ ion. Phys Chem Miner 35(8):467–475. https://doi.org/10.1007/s00269-008-0241-7
Graulis S, Chateigner D, Downs RT, Yokochi AFT, Quirós M, Lutterotti L, Manakova E, Butkus J, Moeck P, Le Bail A (2009) Crystallography open database-an open-access collection of crystal structures. J Appl Crystallogr 42(4):726–729. https://doi.org/10.1107/S0021889809016690
He Q, Wang H, Zhao X, Chen L (2021) Recent advances of transition metal oxalate-based micro- and nanomaterials for electrochemical energy storage: a review. Materials today chemistry. Elsevier, Amsterdam. https://doi.org/10.1016/j.mtchem.2021.100564
Hermanek M, Zboril R, Mashlan M, Machala L, Schneeweiss O (2006) Thermal behaviour of iron(II) oxalate dihydrate in the atmosphere of its conversion gases. J Mater Chem 16(13):1273. https://doi.org/10.1039/b514565a
Hermanek M, Zboril R, Medrik I, Pechousek J, Gregor C (2007) Catalytic efficiency of iron(III) oxides in decomposition of hydrogen peroxide: competition between the surface area and crystallinity of nanoparticles. J Am Chem Soc 129(35):10929–10936. https://doi.org/10.1021/ja072918x
Katada M, Ogimoto T (2000) Mössbauer spectroscopy of iron oxides and related compounds. J Radioanal Nucl Chem 246(1):7–14
Klencsár Z, Kuzmann E, Vértes A (1996) User-friendly software for Mössbauer spectrum analysis. J Radioanal Nucl Chem Art 210(1):105–118. https://doi.org/10.1007/BF02055410
Koga N, Sato Y (2011) Formation and transformation kinetics of amorphous iron(III) oxide during the thermally induced transformation of ferrous oxalate dihydrate in air. J Phys Chem A 115(2):141–151. https://doi.org/10.1021/jp110407n
Kolezynski A, Malacki A (2009) First principles studies of thermal decomposition of anhydrous zinc oxalate. J Therm Anal Calorim 96(2):645–651. https://doi.org/10.1007/s10973-008-9494-0
Kondrashev YuD, Bogdanov VS, Golubev SN, Pron’ GF (1985) Crystal structure of the ordered phase of zinc oxalate and the structure of anhydrous Fe2+, Co2+, Ni2+, Cu2+, and Zn2+ oxalates. J Struct Chem 26(1):74–77. https://doi.org/10.1007/BF00747766
Kopp J, Novak P, Kaslik J, Pechousek J (2019) Preparation of magnetite by thermally induced decomposition of ferrous oxalate dihydrate in the combined atmosphere. Acta Chim Slov 66(2):455–465. https://doi.org/10.17344/acsi.2019.4933
Li Y, Gao G, Wang Q, Zhang K, Yao Y (2021) Synthesis and electrochemical performances of iron oxalate-multiwalled carbon nanotubes composite anode for lithium-ion batteries. In: IOP Conference Series: Earth and Environmental Science. Vol. 692. IOP Publishing Ltd. https://doi.org/10.1088/1755-1315/692/2/022073
Lisníková S, Kopp J, Vrba V, Novák P (2022) Single-phase precursors for the preparation of spinel ferrites via oxalate route: the study of cobalt ferrite synthesis. Chem A Eur J. https://doi.org/10.1002/chem.202104331
Lutterotti L (2010) Total pattern fitting for the combined size-strain-stress-texture determination in thin film diffraction. Nuclear Instruments and Methods in Physics Research, Section B: Beam Interactions with Materials and Atoms 268(3–4):334–340. https://doi.org/10.1016/j.nimb.2009.09.053
Macklen ED (1967) Influence of atmosphere on the thermal decomposition of ferrous oxalate dihydrate. J Inorg Nucl Chem 29(5):1229–1234. https://doi.org/10.1016/0022-1902(67)80362-2
Mohamed MA, Galwey AK, Halawy SA (2005) A comparative study of the thermal reactivities of some transition metal oxalates in selected atmospheres. Thermochim Acta 429(1):57–72. https://doi.org/10.1016/j.tca.2004.08.021
Momma K, Izumi F (2011) VESTA 3 for three-dimensional visualization of crystal, volumetric and morphology data. J Appl Crystallogr 44(6):1272–1276. https://doi.org/10.1107/S0021889811038970
Naumov DY, Podberezskaya NV, Boldyreva EV, Virovets AV (1996) Crystal-chemical analysis of the structures of oxalic acid and its salts Mx(C2O4)y nH2O (N=0−3). J Struct Chem 37:3(480–503). https://doi.org/10.1007/BF02578605
Novák P, Procházka V, Stejskal A (2022) Universal drive unit for detector velocity modulation in Mössbauer spectroscopy. Nucl Inst Methods Phys Res Sect A Accel Spectrom Detectors Assoc Equip. https://doi.org/10.1016/j.nima.2022.166573
Ogasawara H, Koga N (2014) Kinetic modeling for thermal dehydration of ferrous oxalate dihydrate polymorphs: a combined model for induction period-surface reaction-phase boundary reaction. J Phys Chem A 118(13):2401–2412. https://doi.org/10.1021/jp500619q
Procházka V, Novák P, Vrba V, Stejskal A, Dudka M (2020) Autotuning procedure for energy modulation in Mössbauer spectroscopy. Nucl Instrum Methods Phys Res Sect B 483(November):55–62. https://doi.org/10.1016/j.nimb.2020.08.015
Smrčka D, Procházka V, Novák P, Kašlík J, Vrba, V (2016) Iron oxalate decomposition process by means of Mössbauer spectroscopy and nuclear forward scattering. In: AIP Conference Proceedings, 1781:020012. American Institute of Physics Inc. https://doi.org/10.1063/1.4966008
Song T, Gao G, Cui D, Wang C, Zhang H, Liang F, Yang B, Zhang K, Yao Y (2023) Achieving ultrastability and efficient lithium storage capacity with high-energy iron(II) oxalate anode materials by compositing Ge nano-conductive sites. Nanoscale 15(6):2700–2713. https://doi.org/10.1039/d2nr06422g
Stejskal A, Procházka V, Dudka M, Vrba V, Kočiščák J, Šretrová P, Novák P (2023) A dual Mössbauer spectrometer for material research, coincidence experiments and nuclear quantum optics. Meas J Int Meas Confed. https://doi.org/10.1016/j.measurement.2023.112850
Tominaka S, Cheetham AK (2014) Intrinsic and extrinsic proton conductivity in metal-organic frameworks. RSC Adv. https://doi.org/10.1039/C4RA11473F
Zhang K, Li Y, Wang Y, Yuan M, Dai Y, Yao Y (2019) Study on the morphologies and electrochemical properties of the iron oxalate/graphene sheet composite with different polymorphs. Mater Lett 238(March):187–190. https://doi.org/10.1016/j.matlet.2018.12.008
Zhang K, Xu R, Wei R, Li Y, Wang Y, Zhang Y, Dai Y, Yao Y (2020) Tunable polymorph and morphology synthesis of iron oxalate nanoparticles as anode materials for lithium-ion batteries. Mater Chem Phys 243(March):122676. https://doi.org/10.1016/j.matchemphys.2020.122676
Zhou W, Tang K, Zeng S, Qi Y (2008) Room temperature synthesis of rod-like FeC2O42H2O and its transition to maghemite, magnetite and hematite nanorods through controlled thermal decomposition. Nanotechnology 19(6):065602. https://doi.org/10.1088/0957-4484/19/6/065602
Deyrieux R, Peneloux A (1969) Divalent metal oxalates. I. Crystal structure of two allotropic forms of dihydrated ferrous oxalate. Bull. Soc. Chim. Fr. 8:2675–2681
Acknowledgements
The authors gratefully acknowledge the financial support from the internal IGA grant of Palacký University (IGA_PrF_2023_003). The authors would like to thank Vlastimil Vrba for his help with Rietveld refinement.
Funding
Open access publishing supported by the National Technical Library in Prague.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
On behalf of all authors, the corresponding author states that there is no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Heger, V., Kopp, J., Procházka, V. et al. Polymorphism of anhydrous iron(II) oxalate. Chem. Pap. 78, 13–22 (2024). https://doi.org/10.1007/s11696-023-03122-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11696-023-03122-6