Abstract
We provide proof-of-concept for the innovative method of 2,6-dichloroindophenol (DCPIP) for measuring the antioxidant activity of plant extracts. Antioxidant content can be determined using the standard DCPIP test and compare the results with the DPPH results as conventional method. DCPIP operates on the premise that the deep blue color of the oxidized dye is reduced to an invisible solution. Ascorbic acid’s antioxidant activity was measured by DCPIP at different times (from 1 to 60 min) and was concentration-dependent, with the maximum activity being at 400 g/mL. In addition, when compared to other incubation durations, the ascorbic acid standard, a natural antioxidant, gave the maximum activity within the first five minutes of incubation with DCPIP. DCPIP is a marker of antioxidant activity both against vitamin C and plant extracts. The DCPIP approach is quick and unaffected by pH variation. The stability of the DCPIP reagent over time (5 and 30 min) and color reduction by ascorbic acid as a natural antioxidant standard were demonstrated using a straightforward and quick method.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Oxidative stress is defined as an imbalance between the processes of producing reactive oxygen species (ROS) and getting rid of them, which happens because of an imbalance between antioxidants and prooxidants in the body. Antioxidants are substances that can prevent the chemical reaction of oxidation from producing harmful free radicals, which can lead to the degradation of organic compounds. They are commonly added to industrial products like polymers and lubricants to extend their usable lifetimes (Klemchuk 2000). In food, antioxidants are used to prevent spoilage, particularly the rancidification of oils and fats. In cells, antioxidants like glutathione and enzyme systems like superoxide dismutase can prevent damage from oxidative stress (Helberg and Pratt 2021). While only vitamins A, C, and E are dietary antioxidants, other dietary compounds have been labeled as antioxidants despite limited evidence of their antioxidant properties in vivo.
Antioxidants are molecules that inhibit the oxidation of other molecules. Oxidation is a chemical process that produces free radicals, which can damage cells and contribute to various diseases, including cancer, cardiovascular disease, and neurodegenerative disorders. Antioxidants counteract the harmful effects of free radicals by neutralizing them and preventing further damage. Oxidative stress occurs when there is an imbalance between the production of reactive oxygen species (ROS) and the body's ability to neutralize them with antioxidants (Martemucci et al. 2022). There are various methods for detecting antioxidants, including chemical assays, electrochemical assays, and chromatographic techniques. These methods provide information about the quantity and activity of antioxidants in different biological matrices, which can be used for the diagnosis and treatment of various diseases (Hohtola 2010; Pinchuk et al. 2012).
The biological bioactivities of Rosmarinus officinalis L. extracts, including their hepatoprotective, antifungal, insecticide, antioxidant, and antibacterial properties, have been the subject of several investigations. It is well recognized that phenolic chemicals play a major role in how biologically active rosemary is Nieto et al. (2018). Moreover, Chilli pepper provide moderate to high concentrations of phytochemicals, such as neutral phenolics and flavonoids, which are essential antioxidants in a plant-based diet and have health advantages above and beyond basic nutrition (Azlan et al. 2022).
A number of analytical techniques are used to evaluate the stability of pure compounds, with high-performance liquid chromatography being the most popular because it consistently produces accurate results even when the material being analyzed is present in low amounts. The radical-scavenging techniques DPPH (2,2'-diphenyl-1-picrylhydrazyl) and ABTS (2,2'-azinobis-(3-ethylbensothiazoline)-6-sulfonic acid), the ferric reducing antioxidant capacity method (FRAP), and the oxygen radical absorbance capacity method (ORAC) have all been used to assess the antioxidant activity in plant extracts. The assays using DPPH and ABTS radicals among them are easier to use and produce more consistent findings (Buenguer 2006; Li et al. 2008; Shanab et al. 2011; Shalaby et al. 2016).
Also, Moniruzzaman et al. (2012) reported that various methods have been developed to detect and quantify antioxidants, including the DPPH, the FRAP, and the ORAC assays. These assays rely on different principles to measure the ability of antioxidants to scavenge free radicals or reduce oxidized compounds. The choice of the assay depends on the type of antioxidant being measured, as well as the sample matrix and desired sensitivity. (Munteanu and Apetrei 2021). The creation of innovative antioxidant detection techniques, including electrochemical and biosensor-based methods, has recently attracted increasing interest. These techniques are appropriate for a variety of applications due to their excellent sensitivity, selectivity, and quick analytical times (Dapkevicius et al. 2001) Overall, the development of reliable and accurate antioxidant detection methods is critical for the evaluation of the antioxidant capacity of foods and supplements as well as the identification of potential therapeutic agents for the prevention and treatment of oxidative stress-related diseases (Frankel and Finley 2008; Pinchuk et al. 2012).
2,6-dichlorophenolidophenolate (DCPIP) is a typical redox indicator dye especially for ascorbic acid (Prantl et al. 2020) and functions as the oxidizing agent in a quick in-line electron transfer process with antioxidants (Merola et al. 2009). Additionally, the color of this dye is blue, but once it activates with an antioxidant substance, it becomes colorless. In an animal model of human melanoma, DCPIP has the potential to be used as a pro-oxidant chemotherapeutic drug that targets human cancer cells. DCPIP-induced cancer cell death is caused by the reduction in intracellular glutathione and the induction of oxidative stress (Elbehery et al. 2019). The pKa of this dye is about 5.90. Additionally, a reported redox potential of + 217 mV has been generated (Dawson et al. 1986).
The current work aims to develop and validate a new method using 2,6-dichlorophenolidophenolate (DCPIP) as an oxidizing agent (reagent) to evaluate the antioxidant activity of different natural extracts and compare it with conventionally used methods (DPPH and KMnO4).
Materials and methods
Chemicals and reagents
Pure ethanol, methanol, and ethyl acetate were purchased from E. Merck Co. (Darmstadt, Germany). 2,6-Dichlorophenolindophenol (DCPIP), sulforhodamine, 2,2 diphenyl-1-picrylhydrazyl (DPPH), potassium permanganate (KMnO4), bisodium carbonate, and ascorbic acid were purchased from Sigma-Aldrich (St. Louis, MO, USA).
Collection of plant samples
Leaves of Rosmarinus officinalis L. (rosemary) and fruits of Chilli pepper (red chilli) were collected from the local market, Giza, Egypt during spring season 2023.
Extraction of active ingredients
The collected herbal samples (leaves from rosemary and fruits from chilli) were air-dried and then, ground to a fine powder. 50 g of the dried powder was subjected to extraction with aqueous methanol (50%), according to Zhang et al. (2018).
Antioxidant activity
DPPH radical scavenging activity
The scavenging effect of successive extracts from plant samples was determined by the method of Yen and Chen (1995). The absorbance of all the sample solutions was measured at 517 nm using Genway spectrophotometer. The percentage (%) of inhibition activity was calculated as follows: % Inhibition = [(AbsC–AbsE)/AbsC] × 100, where: AbsC is DPPH solution (0.16 mM) absorbance; and AbsE is extract absorbance.
KMnO4 as a non-radical scavenging activity
The scavenging effect of successive extracts from plant samples was determined by the method of Gaber et al. (2021). The absorbance of all the sample solutions was measured at 514 nm using Genway spectrophotometer. The percentage (%) of inhibition activity was calculated as follows: % Inhibition = [(AbsC–AbsE)/AbsC] × 100, where: AbsC is KMnO4 solution (0.16 mM) absorbance; and AbsE is extract absorbance.
DCPIP scavenging assay
The scavenging effects of natural extracts were determined by the new method as follows: 1.0 ml of 0.05 g/100 ml DCPIP solution (soluble in 0.04% sodium bicarbonate) or 0.05 g/100 ml DCPIP solution (soluble in aqueous ethanol solution, 70%) was added to a test tube containing a 1.0 ml aliquot of the sample (with different concentrations). The mixture was vortexed for 1 min and kept at room temperature for different times (from 1 to 30 min) in the dark. The absorbances of all the sample solutions were measured at 600 nm using Genway spectrophotometer. The percentage (%) of inhibition activity was calculated as follows: % Inhibition = [(AbsC–AbsE)/AbsC] × 100, where: AbsC is DCPIP solution (0.05%) absorbance; and AbsE is extract absorbance.
Effect of pH on DCPIP absorption and activity
The effect of different pH values (3, 5, 7, 9, and 11) on the DCPIP color and activity was studied by adding 1.0 ml of DCPIP solution to a tube containing distilled water with a different pH value, and after incubation at room temperature, the absorbance was measured at 600 compared with the DCPIP solution as native (control).
Statistical analysis
All the data are expressed as the mean ± standard deviation. A statistical comparison was performed via a one-way analysis of variance followed by Duncan’s multiple range test (DMRT). P-values of less than 0.05 (P ≤ 0.05) were considered significant.
Results and discussion
Antioxidant activity using DCPIP at different times
The majority of past researchers have used the DCPIP titrimetric method as a vitamin C content indicator, especially for ascorbic acid. Although it was thought that the DCPIP titrimetric method is successful only for ascorbic acid, as well as its limitation to colored fruit only, as reported by Abeysuriya et al. (2020), the DCPIP was applied as a photometric method. The antioxidant activity of ascorbic acid at different concentrations was evaluated using DCPIP, DPPH (radical), and KMnO4 (non-radical) scavenging methods. Then, DCPIP was used as an antioxidant activity indicator against pepper, chilli, and rosemary plant extracts.
The obtained results in Table 1 revealed that the antioxidant activity of ascorbic acid determined using DCPIP at different times (from 1 min until 60 min) was concentration-dependent and recorded the highest activity at 400 μg/mL. Moreover, the obtained data illustrated that the incubation of ascorbic acid as a natural antioxidant standard with DCPIP gave the highest activity during 5 min when compared with other incubation times, as shown in Table 2, as an antioxidant activity indicator not only against vitamin C but also against plant extracts.
Comparison of the antioxidant activity of ascorbic acid using DPPH, KMnO4 and DCPIP
The obtained data in Tables 2 and 3 proved that the percentage of scavenging activity (antioxidant activity) was directly proportional to the concentration of ascorbic acid in all assays (DPPH, KMnO4 and DCPIP). The highest antioxidant activity was recorded by DPPH and KMnO4 at 30 min by 89.38 and 77.84%, respectively, However, ascorbic acid gave 74.20%. against DCPIP assay at a concentration of 400 μg/mL.
Effect of pH values on DCPIP activity
The effect of changes in pH on DCPIP absorbance was evaluated. As shown in Table 4, the change in the absorbance of different treatments, which range from 0.144 to 0.155 at different pH values, is slightly significant and this led to the possibility of using DCPIP as a good indicator for the detection and quantification of antioxidant activity in all extracts without interference from the pH medium when compared with other antioxidant evaluating assays.
With varying degrees of success, several authors have previously measured the impact of pH on antioxidant activity experiments. ABTS was discovered to be unstable at pH levels higher than 7.4 and stable between pH 3.0 to 6.5 (with an optimal pH at pH 4.5). The literature on pH's impact on DPPH tests is more contentious. Since the first article on using DPPH to determine antioxidant levels, the significance of pH has been examined, and a range of 5.0 to 6.5 has been advised. However, in current practice, this advise is frequently disregarded, and according to various articles, pH is irrelevant for the DPPH assay because organic solvents like methanol are used (Ozgen et al. 2006).
Antioxidant activity of plant extract (chili powder)
Chilli (Capsicum annuum L.) is a valuable source of antioxidants due to its high content of vitamins A, C, and B and lutein. According to the United Nations Food and Agriculture Organisation, chilli is the most important crop in the genus Capsicum in the world. Calories, proteins, lipids, carbs, calcium, phosphorus, iron, vitamins A, B, and C, as well as water, are all chemical components of fresh chilli. Additionally, capsanthin, carotenoids, alkaloids, resins, and essential oils are among the alkaloid substances found in chilli. The majority of these ingredients are antioxidant sources. Khuriyati et al. (2022).
Rosemary (Rosmarinus officinalis L.) has received the most attention among herbs and spices as a source of antioxidants. In prior research, rosemary was shown to have comparable patterns of phenolic compounds. Their antioxidant action was mostly due to their carnosic acid, carnosol, and rosmarinic acid constituents (Thorsen and Hildebrandt 2003).
A novel method was used to determine the antioxidant activity of a hydromethanolic extract of red chilli powder and rosemary using DCPIP as an antioxidant activity indicator.
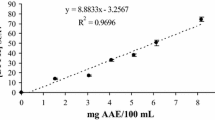
The determination of the antioxidant activity (%) of different concentrations of hydromethanolic extracts of red chilli and rosemary in Table 5 revealed that the 400 μg/mL of both hydromethanolic extracts recorded the highest antioxidant activity; however, the lowest antioxidant activity was recorded by the hydromethanolic extracts of 25 μg/mL. Table 5 clearly indicated that there were no significant differences in antioxidant activity between both methods, DPPH and DCPIP, which assures the possibility of using DCPIP instead of DPPH.
As mentioned in Table 6, correlation coefficient values were obtained among 3 different assays (DCPIP, DPPH, and KMnO4). Results obtained in Table 5 revealed that there is a positive correlation between antioxidant activity and DCPIP and DPPH.
The differences between DPPH and DCPIP as oxidizing agents
Table 7 represented the differences between DPPH and DCPIP as oxidizing agents. The use of the oxidant DCPIP for the determination of antioxidant activity in extracts appears to be a reasonable alternative to published methodologies for assessing antioxidant activity. The DCPIP methodology is rapid, does not affect variable pH, requires minimal amounts of sample, and gives accurate results.
A simple and fast byproduct (as strips) was conducted to prove the stability of the DCPIP reagent over time (5 and 30 min) and color reduction by ascorbic acid as a natural antioxidant standard. As clearly proven in Fig. 1, the DCPIP dye is stable through the examined time and reacts rapidly with antioxidants.
Byproduct as a fast test proven the stability of the DCPIP reagent during the time (5 and 30 min) and color reduction by ascorbic acid as a natural antioxidant standard.
When it comes to its capacity to oxidize vitamin C, the brightly colored reaction known as 2,6-dichloroindophenol, or DCPIP, is highly specific. In neutral and basic solutions, DCPIP is dark blue; in acidic solutions, it is red. The half-reactions are the substances that are engaged in this redox reaction.
So, vitamin C is oxidized by DCPIP in a 2e–/2H+ transfer. Redox reaction with DCPIP provides a quantitative measure of the antioxidant content in a sample, where the solution color will be removed to colorless (Hughes 1983).
The 2,2-diphenyl-1-picrylhydrazyl (DPPH) method incorporates the use of free radicals to quantify the antioxidant properties of compounds and evaluate their potential as hydrogen sources or free-radical scavengers (Fig. 2). The removal of DPPH, which would be a stabilized free radical, is connected to the DPPH testing technique. An odd electron combines with the free radical DPPH to produce a significant absorbance at 517 nm, or a purple color. When an antioxidant combines with DPPH, it produces DPPHH, which contains more hydrogen than DPPH and has a lower absorbance. The DPPH.+It is more radical than the DPPH-H form because when the amount of electrons absorbed rises, it decolorizes or takes on a yellow hue. The lower capacity is affected by decolorization. Decolorization has a substantial impact on reducing capacity. The lower state of diphenylpicrylhydrazine is created as soon as the DPPH solutions are mixed with the hydrogen atom source, losing its violet color. Decolorization has a substantial impact on reducing capacity. The lower state of diphenylpicrylhydrazine is created as soon as the DPPH solutions are mixed with the hydrogen atom source, losing its violet color (Baliyan et al. 2022).
Conclusion
The DCPIP technique was demonstrated in this study that for measuring the antioxidant capacity of substances to serve as hydrogen suppliers can be a quick, easy, and affordable approach for determining antioxidant properties. The decrease in oxidized DCPIP is used in the DCPIP analysis technique. The DCPIP interacts with hydrogen donor compounds and led to decreases the DCPIP blue color. Antioxidants combine with DCPIP and the existence of a hydrogen source (an antioxidant), resulting in the reduction in DCPIP to DCPIPH2 and a reduction in DCPIP absorbance.
Data availability
All data are available under request.
References
Abeysuriya HI, Bulugahapitiya VP, Loku Pulukkuttige J (2020) Total vitamin C, ascorbic acid, dehydroascorbic acid, antioxidant properties, and iron content of underutilized and commonly consumed fruits in Sri Lanka. Int J Food Sci. https://doi.org/10.1155/2020/4783029
Azlan A, Sultana S, Huei CS, Razman MR (2022) Antioxidant, anti-obesity, nutritional and other beneficial effects of different chili pepper: a review. Molecules 27(3):898. https://doi.org/10.3390/molecules27030898
Baliyan S, Mukherjee R, Priyadarshini A, Vibhuti A, Gupta A, Pandey RP, Chang CM (2022) Determination of antioxidants by DPPH radical scavenging activity and quantitative phytochemical analysis of Ficus religiosa. Molecules. https://doi.org/10.3390/molecules27041326
Buenguer J, Ackermann H, Jentzsch A, Mehling A, Pfitzner I, Reiffen KA, Schroeder KR, Wollenweber U (2006) An inter laboratory comparaison of methods used to assess antioxidant potentials. Int J Cosmet Sci. https://doi.org/10.1111/j.1467-2494.2006.00311
Dapkevicius A, van Beek TA, Niederländer H (2001) Evaluation and comparison of two improved techniques for the on-line detection of antioxidants in HPLC eluates. J Chrom A. https://doi.org/10.1016/S0021-9673(01)00548-9
Dawson RMC, Elliott DC, Elliottv WH, Jones KM (1986) Data for biochemical research, 3rd edn. Oxford Science Publications, Oxford, pp 0-19-855358–7
Elbehery NHA, Abd El-Galil EA, Kamel AH, Elsayed AE, Hassan SSM (2019) Novel potentiometric 2,6-dichlorophenolindo-phenolate (DCPIP) membrane-based sensors: assessment of their input in the determination of total phenolics and ascorbic acid in beverages. Sensors Basel. https://doi.org/10.3390/s19092058
Frankel EN, Finley JW (2008) How to standardize the multiplicity of methods to evaluate natural antioxidants. J Agric Food Chem. https://doi.org/10.1021/jf800336p
Gaber NB, El-Dahy SI, Shalaby EA (2021) Comparison of ABTS, DPPH, permanganate, and methylene blue assays for determining antioxidant potential of successive extracts from pomegranate and guava residues. Biomass Conv Bioref. https://doi.org/10.1007/s13399-021-01386-0
Helberg J, Pratt DA (2021) Autoxidation vs. Antioxidants—the fight for forever. Chem Soc Rev. https://doi.org/10.1039/D1CS00265a
Hohtola A (2010) Bioactive compounds from northern plants. Adv Exp Med Biol. https://doi.org/10.1007/978-1-4419-7347
Hughes DE (1983) Titrimetric determination of ascorbic acid with 2,6-dichlorophenol indophenol in commercial liquid diets. J Pharm Sci. https://doi.org/10.1002/jps.2600720208
Khuriyati N, Sukartiko AC, Alfiani RN (2022) Non-destructive measurement of antioxidant activity and water content in chili powder (capsicum annuum L.) using near-infrared spectroscopy. Int Food Res J, https://doi.org/10.47836/ifrj.29.2.10
Klemchuk PP (2000) Antioxidants. Ullmann’s Encycl Ind Chem. https://doi.org/10.1002/14356007.a03_091.ISBN3527306730
Li HB, Wong CC, Cheng KW, Chen F (2008) Antioxidant properties in vitro and total phenolic contents in methanol extracts from medicinal plants. LWT Food Sci Technol. https://doi.org/10.1016/j.lwt.2007.03.011
Martemucci G, Costaglila C, Mariano M, D’andrea L, Napolitano P, D’Alessandro AG (2022) Free Radical Properties. Oxygen, Source and Targets, Antioxidant Consumption and Health. https://doi.org/10.3390/oxygen2020006
Merola ET, Catherman AD, Yehi JB, Strein TG (2009) Determination of total antioxidant capacity of commercial beverage samples by capillary electrophoresis via in-line reaction with 2,6—dichlorophenolindophenol. J Agric Food Chem. https://doi.org/10.1021/jf901214r
Moniruzzaman M, Khalil MI, Sulaiman SA, Gan SH (2012) Advances in the analytical methods for determining the antioxidant properties of honey: a review. Afr J Trad, Compl and Altr Med,. https://doi.org/10.4314/ajtcam.v9i1.5
Munteanu IG, Apetrei C (2021) Analytical methods used in determining antioxidant activity: a review. Int J Mol Sci. https://doi.org/10.3390/ijms22073380
Nieto G, Ros G, Castillo J (2018) Antioxidant and antimicrobial properties of rosemary (Rosmarinus officinalis, L.): A Review. Med Basel. 5(3):98. https://doi.org/10.3390/medicines5030098
Ozgen M, Reese RN, Tulio AZ Jr, Scheerens JC, Miller AR (2006) Modified 2,2- azino-bis-3-ethylbenzothiazoline-6-sulfonic acid (ABTS) method to measure antioxidant capacity of selected small fruits and comparison to ferric reducing antioxidant power (FRAP) and 2,20 -diphenyl-1-picrylhydrazyl (DPPH) methods. J Agri Food Chem 56:1151–1157
Pinchuk I, Shoval H, Dotan Y, Lichtenberg D (2012) Evaluation of antioxidants: scope, limitations and relevance of assays. Chem Phys Lipid. https://doi.org/10.1016/j.chemphyslip.2012.05.003
Prantl L, Eigenberger A, Gehmert S, Haerteis S, Aung T, Rachel R, Jung EM, Felthaus O (2020) (2020) Enhanced resorption of liposomal packed vitamin c monitored by ultrasound. J Clin Med 9(6):1616. https://doi.org/10.3390/jcm9061616
Shalaby EA, Mahmoud GI, Shanab MM (2016) Suggested mechanism for the effect of sweeteners on radical scavenging activity of phenolic compounds in black and green tea. Front Life Sci 9(4):241–251
Shanab SMM, Ameer MA, Fekry AM, Ghoneim AA, Shalaby EA (2011) Corrosion resistance of magnesium alloy (AZ31E) as orthopaedic biomaterials in sodium chloride containing antioxidantly active compounds from Eichhornia crassipes. Int J Electrochem Sci 6:3017–3035
Thorsen MA, Hildebrandt KS (2003) Quantitative determination of phenolic diterpenes in rosemary extracts: aspects of accurate quantification. J Chromatogr A. https://doi.org/10.1016/s0021-9673(03)00487-4
Yen GC, Chen HY (1995) Antioxidant activity of various tea extracts in relation to their antimutagenecity. J Agric Food Chem. https://doi.org/10.1021/jf00049a007
Zhang QW, Lin LG, Ye WC (2018) Techniques for extraction and isolation of natural products: a comprehensive review. Chin Med. https://doi.org/10.1186/s13020-018-0177-x
Funding
Open access funding provided by The Science, Technology & Innovation Funding Authority (STDF) in cooperation with The Egyptian Knowledge Bank (EKB).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare that they have no competing interests.
Consent for publication
All authors read and approved the final manuscript.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Shalaby, E.A., Aboul-Enein, A.M., Sayed, N.M. et al. Validation of an alternative quantitative method for determination of antioxidant potential in Rosmarinus officinalis L. and Chilli pepper. Chem. Pap. 78, 275–282 (2024). https://doi.org/10.1007/s11696-023-03076-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11696-023-03076-9