Abstract
A recently proposed combination of headspace liquid-phase microextraction with an optical immersion probe (HS-LPME-OIP) was adapted for the spectrophotometric determination of ammonium. The developed method is based on the conversion of ammonium ions to ammonia in a 0.15 M sodium hydroxide solution, followed by the absorption of ammonia by 200 µL of Nessler's reagent solution preliminary diluted with distilled water (1:16.7). The extracting phase, in which OIP was immersed, was placed into vial fixed in the headspace above the sample solution in a hermetically sealed vessel. The analytical signal was monitored for 30 min at 400 nm. The duration of the analysis can be flexibly adjusted and, if necessary, reduced to 5 min. The level of permissible concentrations of some interfering substances, including calcium, magnesium, iron and some organic compounds, was significantly reduced. The LOD was equal to 0.20 mg L−1, and the linear calibration range was from 0.70 to 15 mg L−1. The RSD ranged from 2.5 to 5.2%. The developed method was successfully applied to the analysis of natural waters, fertilizers and CRM samples.
Graphical abstract

Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Ammonium content is an important parameter for water quality monitoring. In nature, it occurs as a result of the decomposition of nitrogenous organic substances. Depending on the pH of natural water, ammonium exists in both ionized (ammonium, NH4+) and more toxic non-ionized (ammonia, NH3) forms. Both forms are readily converted into each other, with the ratio of ammonia to ammonium, which is largely dependent on the pH, salinity and temperature. Ammonium predominates when the pH is below 8.75. Most waters have a pH range, where this compound is in ionic form (Molins-Legua et al. 2006). High ammonia levels negatively affect fish populations in natural waters (Milne et al. 2000). In small concentrations, ammonium is an important source of nitrogen for algae. However, excess ammonia exposure in natural waters causes excessive algal blooms, which upset the balance of the ecosystem (Constable et al. 2003). Fertilizers and industrial wastes are the main sources of water pollution with ammonium ions. There are no WHO limits for ammonium content in natural waters. European legislation for surface water samples (EEC/98/83) is 1.5 mg/L as ammonium, and for wastewater samples (EEC/91/271) it is 20 mg/L expressed as total nitrogen (Molins-Legua et al. 2006).
Spectrophotometry is the most commonly used and accepted standard method for the determination of ammonium in water bodies. Spectrophotometric methods used to determine ammonium include the Nessler's method, the indophenol blue method, and modifications such as the indothymol method and the Roth method (Molins-Legua et al. 2006). Some colorimetric and spectrophotometric methods based on more specific reactions were also proposed over the last few years (Amirjani and Fatmehsari 2018; Guspita and Ulianas 2020; Cho et al. 2017). Their sensitivity is on the level of hundreds of mg L−1; however, colorimetric sensor showed much better sensitivity with a linear range starting from 10 mg L−1 (Cho et al. 2017). More research works are devoted to the compounds that contain nitrogen. One of the most popular methods is various microextraction techniques (Altunay et al. 2023; Nemati et al. 2022). An ion-selective electrode method is the cheapest and most environmentally friendly approach. However, commercially available ion-selective electrodes are not suitable for low concentrations, suffer from amine interference and are not applicable to some samples, such as sea or mine water.
Nessler's reagent is a solution consisting of mercury(II) iodide and potassium iodide in a strongly alkaline solution that reacts with ammonium to form a yellow–brown complex. This is the basis for the spectrophotometric determination of ammonium. The consistency and method of Nessler’s reagent preparation significantly affect its properties (Hu et al. 2020). Therefore, it is recommended to use a standard solution purchased from the manufacturer.
The Nessler's method is widely used in environmental analysis. It is one of the most selective among other spectrophotometric methods and is mainly used for ammonium determination in fresh and moderately polluted waters. When analyzing sea- or wastewater, additional sample pre-treatment steps are required, such as distillation, to eliminate the matrix effects due to sample turbidity, color, or the presence of high concentrations of metal ions such as magnesium(II), calcium(II) and iron(III). The procedure using Nessler's reagent is slower than other spectrophotometric methods. In addition, this reagent is extremely toxic (Zhu et al. 2019).
Many different studies have been carried out to improve the classical Nessler's method. Below, attention will be paid only to the last of them. It has been proposed to use masking agents and some separation procedures in order to eliminate the disadvantages mentioned above. Often salts of citric and other hydroxy acids are used to mask magnesium(II), calcium(II) and other metal ions. A combination of sodium citrate, sodium tartrate and polyvinyl alcohol has been proposed to suppress matrix interference (Jeong et al. 2013). However, interference from iron(II), magnesium(II), high concentration of chloride, free chlorine is only reduced, but not completely eliminated in this way. Many attempts have been made to automate the determination of ammonium with Nessler's reagent. A low-cost portable flow system with an optoelectronic detector has been proposed (Kolacinska and Koncki 2014). The problem of precipitate deposition was eliminated by washing the flow manifold with 0.1 M HCl. At the same time, the LOD of the method was about 1.8 mg L−1 and matrix effect was present.
The idea of simultaneous preconcentration and separation of ammonia using its volatility was implemented in different approaches. A simple, fast and reliable flow injection method based on membrane separation is often used to determine ammonium (Timofeeva et al. 2015). It is based on the capture of ammonium with an alkaline solution, followed by the formation of gaseous ammonia. Next, ammonia is transported in a stream of nitrogen to the gas diffusion cell of the flow-injection system, where it penetrates through a hydrophobic porous membrane and is absorbed by an acceptor solution containing an acid–base indicator. The second approach to separation/preconcentration of ammonium is based on the headspace single-drop microextraction followed by colorimetric detection of the reaction product. As an example, the “lab-in-syringe” concept was proposed for automated online membraneless gas separation of ammonia (Giakisikli and Anthemidis 2018). The adoption of reduced and increased pressure conditions, which were created by two independent micro-syringe pumps connected to each other inside the common headspace area of the syringe barrels, resulted in increased extraction rates. However, such flow analysis systems are relatively complex and require programming and non-standard components assembled by experienced users.
Some other articles are devoted to flow injection analysis and its combination with gas diffusion techniques for ammonium determination (Putri et al. 2022). Such systems are interesting from the point of automation, but limited in sensitivity due to flow rate limitations. In addition, they also require specialized equipment.
For the determination of ammonium, a simple and easy-to-manufacture paper-based microfluidic analytical instrument combined with Nessler's reagent was proposed. It requires less reaction time than the classical method and is not affected by the sample matrix, but has a higher detection limit (3.1 mg L−1 in terms of total nitrogen) (Phansi et al. 2016). Nessler's reagent can also be used to detect different forms of nitrogen, including ammonium, nitrite and nitrate, after ion chromatographic separation. The detection limit for ammonium ion was 1 mg L−1 (Niedzielski et al. 2006).
We have recently proposed to combine a fiber optic probe with headspace microextraction using a specially designed vial to hold the extracting phase, completely solving the problem of solvent drop stability at the end of microsyringe or in the hole of OIP (Tamen and Vishnikin 2021; Skok et al. 2022). This provides a flexible solution for online monitoring of the kinetics of chemical processes and improves analysis throughput. It also eliminates the transfer of the analyzed solution into the cuvette, which greatly simplifies and improves the accuracy and reliability of the analytical procedure.
This paper presents a new miniaturized HS-LPME-OIP method based on the conversion of ammonium to ammonia in an alkaline solution, followed by its absorption with a solution of Nessler's reagent. The method allows minimizing the amount of Nessler`s reagent used per determination. Complete separation from the matrix components allows a significant increase in selectivity compared to the classical approach, avoiding the influence of nonvolatile compounds on the determination. The method was successfully applied to determination of ammonium in natural waters, fertilizer and CRM samples.
Experimental
Materials and equipment
All reagents used were of analytical grade purity. Water for conducting experiments was firstly double distilled then boiled up to two thirds of the initial volume. Then it was kept without fresh air access. 1 g L−1 NH4+ solution was prepared by dissolving 2.965 g of NH4Cl (CentralChem, Slovakia) in distilled water and diluting up to 1 L in a volumetric flask. 3 M NaOH (CentralChem, Slovakia) solution was prepared by dissolving 12 g of NaOH in distilled water and diluting up to 100 mL in a volumetric flask. Nessler's reagent was purchased from CentralChem, Slovakia (d = 1.16 g cm−3). The diluted Nessler's reagent was prepared by diluting 16.7 times with distilled water of the commercial reagent containing 0.09 M solution of potassium tetraiodomercuriate(II) (K2HgI4) in 2.5 M potassium hydroxide. 0.01 M and 1 M solutions of possible interfering compounds were prepared by dissolving the appropriate amounts of salts in distilled water and diluting up to 25 mL in a volumetric flask. CombiCheck 10 CRM from Merck, Germany (1.14676.0001), was used to check the accuracy of the method.
Double-pass optical immersion probe with 1 cm path length (Expedeon, UK) connected to a USB 4000 fiber optic spectrometer (Ocean Optics, USA) and DH-2000 UV–VIS-NIR light source (Ocean Optics, USA) was used for analytical signal measurements. OceanView spectroscopy software was used to collect the data. The reaction system was stirred with a magnetic hotplate stirrer (AREX Digital, VWR international GmbH, Germany). RW-0525G Refrig/Heat Bath Circulator (Jeio Tech Co., Korea) was used to heat the reaction mixture.
HS-LPME-OIP procedure for ammonium determination
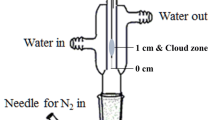
An aliquot of the standard solution of the analyte, corresponding to an ammonium concentration of 0.7–15 mg L−1 in a final volume of 5 mL, was placed in a glass flat-bottomed conical flask together with a stirrer. The flask was fixed on a magnetic stirrer in a circulating water bath connected to a thermostat. The flask was hermetically sealed with a rubber stopper into which an optical immersion probe and a syringe with sodium hydroxide solution were mounted. Also, 200 µL of the diluted Nessler's reagent was placed into a container fixed on the cork bottom. The temperature was maintained at 50 °C. To initiate the release of ammonia, 250 µL of 3 M NaOH was added to the flask with the sample solution. The magnetic stirrer was set to 1000 rpm. The absorbance was measured continuously at 400 nm immediately after the start of stirring for 30 min.
Results and discussion
Optimization of the conditions for ammonium determination by HS-LPME-OIP method
The first step in preconcentration methods based on headspace microextraction is to convert the analyte to volatile derivatives. When determining ammonium, it is customary to convert it to ammonia in a sufficiently alkaline solution. The influence of the concentration of sodium hydroxide solution in the range from 0.075 to 0.3 M on the release of ammonia from the sample solution was studied. The maximum gas evolution was observed at a sodium hydroxide concentration of 0.15 M or more. For further experiments, a sodium hydroxide concentration of 0.15 M was used.
The volume of the acceptor phase was limited to 200 μL, which was the smallest possible volume required to completely fill the path of the optical probe in the acceptor vial. Nessler's reagent was chosen as an absorbing solution and for carrying out the color-forming reaction. Its concentration is crucial for the determination of ammonium. As a result of the reaction with ammonium ions, an orange-yellow substance is formed, which slowly precipitates. The higher the concentration of the Nessler's reagent, the more fully the reaction goes to completion. However, at higher concentrations, the sparingly soluble reaction product precipitates faster, making absorbance measurements less reproducible. It has been established that with preliminary dilution of the Nessler's reagent by 16.7 times with distilled water, the effect of precipitate formation on the results obtained becomes insignificant. Dilution with sodium hydroxide is unsatisfactory, as it accelerates the formation of a precipitate.
The dependence of the analytical signal on the sample volume was studied in the range from 5 to 25 mL. It was found that maximum analytical signal was obtained for 5 mL of the donor phase. At higher volumes, the extraction efficiency decreased that can be explained by the deterioration of the conditions for the mass transfer of analyte between three phases. Therefore, this volume was chosen as an optimal.
To accelerate the release of ammonia from the donor phase, it is necessary to heat the solution. At room temperature, the reaction takes more than one and a half hours to complete. It was found that the rate of ammonia evaporation is accelerated with increasing temperature up to 50 °C, after which the analytical signal begins to decrease. The temperature of 50 °C was chosen as the optimal one.
The sample solution must be agitated to enhance mass transfer in the sample phase and induce headspace convection. It was found that to achieve the maximum analytical signal, the stirring speed should be about 1000 rpm. With a further increase in the stirring rate, the increase in absorbance stops. This indicates that complete equilibrium is established in the system under study, and the total extraction rate depends only on the processes occurring in the acceptor phase.
Determination of ammonium concentration with fixed time method
The recommended time of the reaction with ammonia for the classical method varies from 10 to 30 min (Zhao et al. 2019). The use of an optical probe allows you to conduct accurate and reproducible measurements of absorbance at a certain time. Thus, using the dependence of absorbance on time, you can choose any time that is optimal in terms of sensitivity or extraction time. The kinetic curve has an initial segment where the absorbance does not differ from the blank value (Fig. 1). In a three-phase system, it takes some time to accumulate the required initial amount of ammonia to start the reaction. The fixed time method proved to be well applicable for the kinetic determination of ammonium ions by the HS-LPME-OIP method.
The equations of the calibration graphs for the dependences of absorbance on the concentration of ammonium ions were calculated for fixed times of 5, 10, 15, 20 and 30 min (Table 1). As can be seen from this table, the slope increases with the time. The values of the correlation coefficients remain high for any fixed time. Thus, any fixed time can be taken for analysis. 30 min is preferred for ammonium determination when sensitivity is important. However, this time could be reduced to 5 min to increase the throughput of the analysis.
Interference study
In the case of classical Nessler's reagent method, the main interfering compounds are metal ions such as magnesium(II), calcium(II), iron(II, III), compounds affecting turbidity and color of water samples, sulfide, free chlorine and some organic compounds. Various masking agents were proposed to improve selectivity, but this greatly complicates the determination and does not completely eliminate interference. EDTA can be used for masking some metal ions. However, in this case, it is recommended to use concentrated Nessler`s reagent to ensure the reaction is complete. (Zhu et al. 2019).
The interfering effect was considered absent if the change in absorbance did not exceed 5% compared to the analytical signal for 0.1 mM ammonium ions (Fig. 2). We have studied the influence of several organic compounds that may interfere with the determination. Methanol and ethanol as representatives of alcohols do not interfere at concentrations of 2500 and 3000 times the ammonium content. A 100-fold excess of urea and a 50-fold excess of propylamine do not affect the determination of ammonium. Common ions present in the environmental waters, including sulfates, phosphates, sodium and potassium in at least thousand-fold excess, bromides, nitrates, carbonates, nitrites in a 100-fold excess, do not cause interference. Sulfides affect the stability of the Nessler’s reagent at concentrations above 10–6 M in the classical approach. Sulfide interference is completely eliminated in the proposed microextraction procedure.
Metal ions in an alkaline medium, inherent to the Nessler’s reagent, can cause the solution to become cloudy and therefore interfere with the classical Nessler's method. Iron(III) and iron(II) already interfere at concentrations of 0.01 mM, while in the proposed HS-LPME-OIP method their tolerable concentrations rise sharply to 0.5 mM. When using the classical method, magnesium and zinc cause an interfering effect starting at 0.02 mM and 1 mM, respectively. Permissible concentrations of these ions are increased according to the developed procedure up to 0.1 and 5 mM, respectively, which is higher than their usual concentrations in most river waters. Calcium has no effect at a concentration of 10 mM. In the proposed microextraction procedure, this parameter is ten times higher.
Analytical figures of merit
For the classical Nessler's reagent method, absorbance measurements are usually carried out in the range from 400 to 425 nm (Nollet and Gelder 2013). The wavelength of 400 nm was chosen as the analytical wavelength. The analytical performance of the HS-LPME-OIP microextraction procedure, obtained under the optimal conditions, is summarized in Table 2. The limit of detection and the limit of quantification were calculated as the concentration equivalent to three times and ten times the ratio of the standard error of the regression to the slope of the calibration plot, respectively.
Analytical application
The developed HS-LPME-OIP method was applied to the determination of ammonium ions in fertilizer and various types of water. The accuracy of the method was assessed by the recovery test, sample dilution test and using the CRM sample (Table 3). All the spiked levels showed good recoveries from 96.5 to 106%. In all cases, the sample matrix did not cause serious interference in the determination of ammonium by the HS-LPME-OIP method. Due to the high ammonium content, the fertilizer was diluted accordingly. All other samples were analyzed as described above.
Conclusions
The Nessler's method is widely used in analytical chemistry and environmental analysis. However, this reagent is highly toxic due to the presence of mercury salts in it, which contradicts the modern requirements of green chemistry. Also, this method has serious problems with selectivity. To overcome these disadvantages, microextraction methods can be used, including the headspace mode, which has a set of advantages, such as simplicity, low cost, a high degree of separation of volatile analytes from matrix components, and a small volume of extrahent.
In this work, a recently proposed combination of headspace mode of LPME with an optical immersion probe was adapted for the spectrophotometric determination of ammonium. The proposed method is simpler and more reliable than the previously proposed approaches. Compared to the single drop microextraction and approaches, where syringe was replaced by an optical probe, even an inexperienced user can easily and quickly place the acceptor phase into the vial without the risk of losing it during analysis (Skok et al. 2022).
The duration of the analysis can be flexibly adjusted and, if necessary, reduced to 5 min. Online monitoring of the analytical signal with optical probe allowed to make reproducible and accurate measurements of the absorbance at any desired time after the start of the reaction. This improves the throughput of the analysis.
Compared with the classical method, the amount of toxic Nessler's reagent used for the determination was significantly reduced from a few milliliters of a concentrated solution to 200 µL of a pre-diluted reagent, that is, about a hundred times less. It is important that the permissible concentrations of some interfering substances were significantly reduced, which increased the selectivity of the method and made the analysis of waters with a relatively high content of calcium, magnesium, iron and some organic compounds more accurate and reliable. Compared with spectrophotometric and colorimetric methods (Amirjani and Fatmehsari 2018; Guspita and Ulianas 2020), the sensitivity of HS-LPME-OIP method is a couple of orders better. Methods based on flow injection analysis (Timofeeva et al. 2015; Putri et al. 2022) or proposed in the literature modifications of Nessler method (Phansi et al. 2016; Kolacinska and Koncki 2014; Niedzielski et al. 2006) are also less sensitive. Additionally, the proposed method is easy to operate and inexpensive. The developed HS-LPME-OIP method showed good accuracy and reproducibility, which allows you to successfully use it to analyze samples of natural waters and fertilizers.
References
Altunay N, Elik A, Tuzen M, Lanjwani MF, Mogaddam MRA (2023) Determination and extraction of acrylamide in processed food samples using alkanol-based supramolecular solvent-assisted dispersive liquid–liquid microextraction coupled with spectrophotometer: optimization using factorial design. J Food Compost Anal 115:105023. https://doi.org/10.1016/j.jfca.2022.105023
Amirjani A, Fatmehsari DH (2018) Colorimetric detection of ammonia using smartphones based on localized surface plasmon resonance of silver nanoparticles. Talanta 176:242–246. https://doi.org/10.1016/j.talanta.2017.08.022
Cho YB, Jeong SH, Chun H, Kim YS (2017) Selective colorimetric detection of dissolved ammonia in water via modified Berthelot’s reaction on porous paper. Sens Actuators B Chem 256:167–175. https://doi.org/10.1016/j.snb.2017.10.069
Constable M, Charlton M, Jensen F, McDonald K, Craig G, Taylor KW (2003) An ecological risk assessment of ammonia in the aquatic environment. Hum Ecol Risk Assess 9(2):527–548. https://doi.org/10.1080/713609921
Giakisikli G, Anthemidis AN (2018) Automatic pressure-assisted dual-headspace gas–liquid microextraction. Lab-in-syringe platform for membraneless gas separation of ammonia coupled with fluorimetric sequential injection analysis. Anal Chim Acta 1033:73–80. https://doi.org/10.1016/j.aca.2018.06.034
Guspita D, Ulianas A (2020) Optimization of complex NH3 with Cu2+ ions to determine levels of ammonia by UV–Vis spectrophotometer. J Phys Conf Ser 1481:012040. https://doi.org/10.1088/1742-6596/1481/1/012040
Hu CY, Gu Q, Guo H, Yao Y, Yao C, Wang J, Chen J (2020) Possible influences on ammonia nitrogen determination by Nessler’s reagent spectrophotometry. J Nat Sci Res 11(24):11–15. https://doi.org/10.7176/JNSR/11-24-02
Jeong H, Park J, Kim H (2013) Determination of NH4+ in environmental water with interfering substances using the modified Nessler method. J Chem 2013:1–9. https://doi.org/10.1155/2013/359217
Kolacinska K, Koncki R (2014) A novel optoelectronic detector and improved flow analysis procedure for ammonia determination with Nessler’s Reagent. Anal Sci 30:1019–1022. https://doi.org/10.2116/analsci.30.1019
Milne I, Seager J, Mallett M, Sims I (2000) Effects of short-term pulsed ammonia exposure on fish. Environ Toxicol Chem 19(12):2929–2936. https://doi.org/10.1002/etc.5620191213
Molins-Legua C, Meseguer-Lloret S, Moliner-Martinez Y, Campíns-Falcó P (2006) A guide for selecting the most appropriate method for ammonium determination in water analysis. TrAC Trends Anal Chem 25(3):282–290. https://doi.org/10.1016/j.trac.2005.12.002
Nemati M, Tuzen M, Farazajdeh MA, Kaya S, Mogaddam MRA (2022) Development of dispersive solid–liquid extraction method based on organic polymers followed by deep eutectic solvents elution; application in extraction of some pesticides from milk samples prior to their determination by HPLC-MS/MS. Anal Chim Acta 1199:339570. https://doi.org/10.1016/j.aca.2022.339570
Niedzielski P, Kurzyca I, Siepak J (2006) A new tool for inorganic nitrogen speciation study: simultaneous determination of ammonium ion, nitrite and nitrate by ion chromatography with post-column ammonium derivatization by Nessler reagent and diode-array detection in rain water samples. Anal Chim Acta 577(2):220–224. https://doi.org/10.1016/j.aca.2006.06.057
Nollet L, De Gelder L (2013) Handbook of water analysis, 3rd edn. CRC Press Taylor & Francis Group, Boca Raton
Phansi P, Sumantakul S, Wongpakdee T, Fukana N, Ratanawimarnwong N, Sitanurak J, Nacapricha D (2016) Membraneless gas-separation microfluidic paper-based analytical devices for direct quantitation of volatile and nonvolatile compounds. Anal Chem 88(17):8749–8756. https://doi.org/10.1021/acs.analchem.6b02103
Putri LA, Sari PM, Sulistyarti H, Sabarudin A, Sulistyo E (2022) Determination of total ammonia nitrogen by gas-diffusion flow injection analysis (GD-FIA)-spectrophotometry using Minnieroot flower (Ruellia tuberosa) as natural reagent. Makara J Sci 26:263–272. https://doi.org/10.7454/mss.v26i4.1344
Skok A, Vishnikin A, Bazel Y (2022) A new approach for sulfite determination by headspace liquid-phase microextraction with an optical probe. Anal Methods 14:3299–3306. https://doi.org/10.1039/d2ay00943a
Tamen A-E, Vishnikin AB (2021) In-vessel headspace liquid-phase microextraction. Anal Chim Acta 1172:338670. https://doi.org/10.1016/j.aca.2021.338670
Timofeeva II, Bulatov AV, Moskvin AL, Kolev SD (2015) A gas-diffusion flow injection method coupled with online solid–liquid extraction for the determination of ammonium in solid samples. Talanta 142:140–144. https://doi.org/10.1016/j.talanta.2015.04.051
Zhao Y, Shi R, Bian X, Zhou C, Zhao Y, Zhang S, Wu F, Waterhouse G, Wu LZ, Tung CH, Zhang T (2019) Ammonia detection methods in photocatalytic and electrocatalytic experiments: how to improve the reliability of NH3 production rates? Adv Sci 6(8):1802109. https://doi.org/10.1002/advs.201802109
Zhu Y, Chen J, Yuan D, Yang Z, Shi X, Li H, Jin H, Ran L (2019) Development of analytical methods for ammonium determination in seawater over the last two decades. TrAC Trends Anal Chem 119:115627. https://doi.org/10.1016/j.trac.2019.115627
Acknowledgements
Yaroslav Bazel and Arina Skok thank the Scientific Grant Agency VEGA of the Ministry of Education of the Slovak Republic and the Slovak Academy of Sciences for their support (Grant No. 1/0177/23). Andriy Vishnikin thanks Government Office of the Slovak Republic for support in the framework of the program «Štipendiá pre excelentných výskumníkov ohrozených vojnovým konfliktom na Ukrajine» (09I03-03-V01-00106).
Funding
Open access funding provided by The Ministry of Education, Science, Research and Sport of the Slovak Republic in cooperation with Centre for Scientific and Technical Information of the Slovak Republic.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
On behalf of all authors, the corresponding author states that there is no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This work was presented at the European Symposium on Analytical Spectrometry ESAS 2022 & 17th Czech-Slovak Spectroscopic Conference held in Brno, Czech Republic on September 4–9, 2022.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Skok, A., Bazel, Y. & Vishnikin, A. A new miniaturized microextraction HS-LPME-OIP procedure for ammonium determination based on Nessler's method. Chem. Pap. 77, 7303–7309 (2023). https://doi.org/10.1007/s11696-023-02903-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11696-023-02903-3