Abstract
A comparison of coagulation with two coagulants, poly-aluminum chloride (PACl) and ferric chloride (FeCl3), followed by microfiltration, was evaluated to obtain a better coagulant for an efficient pretreatment method for make-up water preparation from Danube water for thermal power plants. Efficiency was determined by chloride concentration and retention based on the total suspended solid content of the treated water samples. Results were compared to microfiltration working alone as a chemical-free pretreatment. Addition of PACl resulted in the lowest total suspended solid content (18.0 ± 1.3 mg/L), slightly lower than obtained for microfiltration alone (19.6 ± 2.5 mg/L) and significantly lower than for FeCl3 (25.0 ± 3.3 mg/L). Regarding the retention values, coagulation with PACl followed by microfiltration, microfiltration working alone and coagulation with FeCl3 followed by microfiltration represented retention values of 68%, 66.21%, and 56.89%, respectively. Considering the chloride concentration, it remained constant after microfiltration alone; meanwhile, adding coagulants showed a significant rise, ~ 6.4- and 5.7-times higher than the raw water's value after adding FeCl3 and PACl, respectively. From environmental viewpoint, microfiltration alone is recommended because it can provide a steady flux and low total suspended solid content without additional load of chloride ion which shall be eliminated in the further desalination step.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The power industry requires a large amount of fresh water as make-up water to compensate the losses of the water cycle. Water losses due to the large amount of cooling tower and boilers blowdown water was the main motivation driving the researchers to concentrate on reuse the blowdown water after treatment process. Corrosion and scale are primarily caused by blowdown water; meanwhile, this water can be treated as an appropriate make-up water resource if it is treated properly. Otherwise, the colloids and particles cause the scale and block the pipes (Cote et al. 2004). Without pretreatment, natural water cannot be used in contemporary technological processes. Therefore, the best solution is to choose appropriate treatment processes resulting in purified make-up water and obtaining the necessary quality of the water circulating in the Rankin cycle (Choshnova 2018). Water quality requirements are stringent, challenging power plants' design and operation (Rajaković-Ognjanović et al. 2011). Therefore, the water treatment process for thermal power plants is very significant to meet these requirements, especially environmental ones (Dobrin et al. 2012).
Researchers conducted a theoretical comparison between the pretreatment technologies. Although traditional pretreatment technologies were the most used before, replacing them with membrane processes is a compelling requirement to improve pretreatment quality (Jiang et al. 2017). Membrane technologies showed high effectiveness and capacity, low energy consumption, and ease of operation to produce clean water. Therefore, in recent decades, they have been selected as preferred technologies for water treatment (Ravanchi et al. 2009). However, fouling is the main issue with the membrane, which decreases membrane life, permeation flux, and recovery rate during filtration, and this issue requires frequent cleaning, even chemical cleaning (Kochkodan and Hilal 2015).
Membrane fouling is a severe issue that increases the water treatment cost due to constant cleaning and maintenance. Also, it negatively affects both selectivity and the quality of the permeate flow, directly impacting water production. Consequently, choosing a suitable pretreatment technique is the best solution to control membrane fouling, thus, maintaining permeate flow and ensuring membrane life (Salamon et al. 2018). Conventional methods such as coagulation and flocculation were prevalent in the pretreatment stage to remove particulate matter, which might reduce membrane fouling and enhance membrane filterability in subsequent processes (Ly et al. 2018). M. Xu and coworkers investigated the effect of three coagulants, poly-aluminum chloride (PACl), polymeric ferric sulfate (PFS), and titanium xerogel coagulant (TXC), on the coagulation–ultrafiltration efficiencies in algae-laden water. Results showed that insufficient dosages of coagulants could not significantly eliminate fouling, and coagulation with low dosages could increase turbidity. Coagulants with higher dosages improved the filtration flux to some extent. Among the three coagulants, for treatment in neutral and alkaline water, PACl showed better efficiency in reducing turbidity during the coagulation process and subsequently improving the filtration flux (Wang et al. 2019; Xu et al. 2022). Farahani M. and colleagues studied coagulation–filtration and compared it to ultrafiltration as a pretreatment step to determine their effects on the flux of the desalination step.
Using coagulation–filtration as a combined pretreatment step showed between 25 and 33% improvement in permeate flux of the desalination step, and it is more cost-effective than ultrafiltration (UF) alone (Farahani et al. 2016). Membrane technologies such as microfiltration (MF) have wide implementations in the water pretreatment stage regarding effectively removing colloids and particles. However, natural organic matter (NOM) with small molecular weight would penetrate the MF membrane and lower the permeate quality (Woo et al. 2017). Using coagulants in the pretreatment stage prior to MF would adsorb NOM and enlarge the foulant size, enhancing the MF performance (Kimura et al. 2014; Teng et al. 2020). Due to the superior performance of traditional aluminum and ferric coagulants, PACl has drawn significant attention in NOM removal (Deng et al. 2019; Ma et al. 2015).
Determining the optimum coagulant dosage is effective for NOM removal, improving the treated water quality. Moreover, this proper dosage positively affects the membrane fouling level. Therefore, H. Park and coworkers studied PACl and ferric chloride (FeCl3) at concentration ranges of 10–50 mg/L for the coagulation process in the pretreatment step to determine the best dosage regarding conductivity, turbidity, and total organic carbon (TOC) in blended surface water. PACl at a dosage of 20 mg/L showed the best performance compared to FeCl3 regarding the removal efficiency of TOC, dissolved organic matter (DOC), and turbidity (Park et al. 2021). K. Konieczny and colleagues investigated the effect of coagulation on the UF step. They found that the type of coagulant used affects how susceptible UF membranes are to fouling. Three different coagulants were used at different dosages (FeCl3, Fe2(SO4)3 and Al2(SO4)3). An improvement in water quality, in terms of NOM as TOC removal and restricting membranes' fouling, was noticed after using the coagulation step. For this purpose, using the aluminum coagulant showed the highest efficiency of the process compared to FeCl3. Regarding the flux, adding FeCl3 to the raw water resulted in a considerable increase in the flux value compared to adding Al2(SO4)3 or the UF working alone (Konieczny et al. 2009). S. Ebrahim and coworkers concluded that the coagulation process requires a large amount of chemicals depending on the water source, unlike the membrane technology, which does not rely on chemicals and makes it more environmentally friendly (Ebrahim et al. 1997).
D. Sakol and K. Konieczny studied the effect of coagulation combined with a filtration step before the MF membrane to reduce the negative impact of the fouling phenomenon. They observed that using coagulation with prefiltration followed by MF resulted in a lower TOC water that could be further treated as a Reverse Osmosis feed. This combination enhanced the retention coefficients of solid suspension and contaminants responsible for the colloidal suspension formation (Sakol and Konieczny 2004). Schäfer et al. tested the effect of coagulation on microfiltration regarding the membrane fouling; water samples were taken from Suwannee River and concluded that coagulation with FeCl3 significantly increased fouling of the membrane, which increases the costs of the filtration process (Schäfer et al. 2001). In addition, Judd and Hillis found that choosing appropriate coagulant dosages is the main performance-determining parameter. Therefore, they evaluated the performance of the microfiltration membrane when using FeCl3 with different dosages in the coagulation step and concluded that using low dosages of coagulants causes internal fouling of the membrane, which lowers the filtration efficiency (Howe and Clark 2006; Judd and Hillis 2001). Similarly, J. Kerry and M. Mark studied different coagulants, including ferric sulfate, Alum and PACl, and evaluated their impact on removing DOC and reducing membrane fouling. They found that selecting the optimum dose of the coagulants plays a vital role in controlling filtration performance. Membrane fouling was frequently worse at low dosages of coagulants compared to the treatment with no coagulation. But with enhanced dosages, the coagulation process is better, and the membrane performance is improved (Howe and Clark 2006).
Some studies have shown improved membrane performance regarding the flux or fouling level. Still, others have shown decreased membrane performance when using coagulation followed by microfiltration in freshwater pretreatment (Howe and Clark 2006). Consequently, depending on the reviewed literature, the importance of the pretreatment step during the water treatment process was concluded to concentrate on reducing the chemicals as possible. The literature reviewed here focused on NOM removal finding the optimal dosage of coagulant and membrane fouling but not dealing with the determination of the optimal pH of the water to be treated, adding rate of coagulants, stirring rate, and further chemical load in the treated water. So, our study aims to examine the effect of coagulants in the treated water, determine the chemical load, compare the batch microfiltration and coagulation technologies as a pretreatment step prior to MF using 0.45 μm pore-size membrane and check the effectiveness of these technologies in producing pure water based on the results of the laboratory work. Meanwhile, the effects of the used coagulants on permeate quality regarding the total suspended solid content (TSS) and the chloride concentration and their effect on membrane separation are studied. It should be noted that previous studies have not dealt with the remaining anion concentration in the treated water, only the metal content originating from the coagulant.
This study focuses on the pretreatment technologies to determine the possibilities of eliminating conventional technology and depending only on membrane filtration. Consequently, obtaining a pretreatment technology that is sustainable and environmentally benign is essential. Based on the theoretical comparison between pretreatment technologies, an experimental plan was developed and executed on the Danube water as freshwater. Membrane fouling observations were not the subject of our experiments; long-lasting experiments were out of focus.
Materials and methods
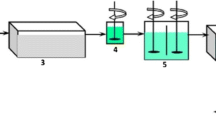
Pretreatment experiments were conducted with two different methods. Coagulation experiments were conducted via jar test with two types of coagulants, PACl and FeCl3. Both coagulants were purchased from VWR Hungary company; PACl (purchased as \({\mathrm{Al}}_{2}\mathrm{Cl}{(\mathrm{OH})}_{5}\) (M = 174.45 g/mol) and FeCl3 (purchased as \({\mathrm{FeCl}}_{3}\cdot 6{\mathrm{H}}_{2}\mathrm{O}\)) (M = 270.30 g/mol). PACl and FeCl3 were tested at different dosages using a stock solution of 1 wt% as a coagulant. First, three initial dosages of PACl (0.1–0.3 mL) were added to 30 mL of Danube water and tested separately. Moreover, three additional dosages of FeCl3 (0.1–0.3 mL) were also tested separately. The addition of coagulants and stirring lasted for 30 s at 400 rpm, as determined previously. Two types of microfiltration membranes were used: 5–13 µm particle retention MF made of cellulose (VWR® Grade 413 Filter Paper, Qualitative) and 0.45 µm pore-size MF made of hydrophilic polyether sulfone (PALL, Supor®-450), both of them were purchased from VWR International Ltd. MF experiments were performed on a universal bench-scale membrane filtration apparatus (CM-Celfa P28; CM-Celfa Membrantrenntechnik AG, Bahnhofstrasse 92, 6423 Sewn, Switzerland), see Fig. 1. Batch filtrations were conducted in a dead-end (5–13) µm particle retention MF and cross-flow mode (0.45 µm pore-size MF). A maximum of 500 mL feed could be used in each experiment. In cross-flow mode, a circulation pump maintained the continuous water recirculation on the feed side, while in batch mode, no circulation was applied. According to the manual, pump delivery rate is 1.81 L/min. The applied pressure was the hydraulic pressure over the atmospheric pressure for both MFs (1.03 bar), which was continuously changing due to feed level change as proceeding the experiments. Membranes with an effective area of 28 cm2 were used for filtration rounds and conditioned with distilled water before use. The experimental plan is illustrated in Fig. 2. TSS content in [mg/L] was measured by a portable ultraviolet (UV) analyzer (PASTEL-UV). As TSS content, the average of five replicates is given. Chloride concentration was measured according to the classical titration method; AgNO3 was used as a reagent. The average concentration of three or five samples is provided as chloride content. Danube water parameters were tested during these experiments and considered a freshwater source; water samples were taken from Műegyetem rkp. Budapest, Hungary (October 10th, 2022). Water characteristics were measured (Table 1); pH and specific electric conductivity (later conductivity) were measured with a 340I type WTW combined pH/conductivity meter.
Results and discussion
Freshwater pretreatment using coagulation
The coagulation process was conducted with two coagulants: PACl and FeCl3, at different dosages, using a stock solution of 1 wt% as a coagulant. TSS content and chloride concentration are appropriate metrics that aid in selecting the most effective pretreatment method. The parameters of the purified water are summarized in Table 2. Based on the TSS measurements, adding 0.2 mL PACl to 30 mL of raw water provided the lowest value of TSS (19.8 ± 3.6 mg/L) and turbidity in the treated water. Meanwhile, 0.3 mL of stock solution of FeCl3 was sufficient for 30 mL of freshwater, providing visibly bigger flocs settled, resulting in the lowest TSS value out of the three dosages, see Fig. 3.
Regarding TSS content, PACl is better than FeCl3 and provides the lowest TSS value in the treated water; thus, PACl is more suitable in the pretreatment step, in agreement with (Wang et al. 2019; Xu et al. 2022). Considering the chloride concentration, any chemical addition increases the chloride concentration in the treated water by at least 16% in the case of 0.1 mL FeCl3 and a maximum of 200% in the case of 0.3 mL PACl compared to the original value of Danube water (35.9 ± 1.4 mg/L). The increased chloride concentration makes the further desalination step more difficult, so it is worth considering the advantage of using MF without coagulation.
Freshwater pretreatment using microfiltration
Freshwater experiments started with two simultaneous rounds of MF to examine the flux during the process (J1 and J2). Flux values were about 4000 L/(m2 h) in the initial phase showing a slightly decreasing trend to 3500 L/(m2 h) at a recovery rate of 95%, which are in the similar range to the values obtained in the seawater experiments (Shaheen and Cséfalvay 2022). Typically, in the case of MF, the recovery rate must be kept within 90–95%; the recovery rate is defined as the ratio of the volume of permeate and feed (Vp/Vf). Voidage measurements helped to determine the mass balance of MF with an error of 3.37 g (equals 0.7%) which is an acceptable value (< 2%). Flux was calculated according to Eq. (1),
where A is the membrane surface [m2]; \(\mathrm{d}V/\mathrm{d}t\) is the flow rate [L/h].
MF parallel experiments were accomplished to check the reproducibility, and the results show the same trend as expected for a batch experiment, Fig. 4.
Parameters of the purified water after the microfiltration step are shown in Table 3. Microfiltration does not influence the chloride concentration; chloride concentration remains constant. MF significantly decreases the initial 58 mg/L TSS content in the permeate to one-third. Compared to the coagulation experiments, the addition of 0.2 mL PACl to 30 mL Danube water resulted in the same TSS content (19.8 ± 3.6 mg/L) as the permeate of MF working alone (19.6 ± 2.5 mg/L).
Freshwater pretreatment using coagulation with further microfiltration
Simultaneously, coagulation with further MF was used as a freshwater pretreatment to compare both processes and examine the possibility of eliminating the coagulation from the pretreatment step or choosing the best coagulants with fewer chemicals. Samples (containing flocs) produced after coagulation were filtered through 5–13 µm particle retention MF membrane followed by 0.45 µm pore-size MF membrane. As shown in Fig. 5, PACl-containing water showed a higher flux verifying that FeCl3 produced bigger flocs resulting lowering flux.
Since the 5–13 µm particle retention MF membrane eliminated the flocs, the remaining particles were filtered by 0.45 µm pore-size MF membrane providing higher flux for water treated with FeCl3, see Fig. 6.
The efficiency of these methods was determined by the TSS content and chloride concentration of the treated water samples, see Tables 3 and 4. Chloride concentration after coagulation with FeCl3 was lower than after coagulation with PACl. Coagulation (PACl) with further MF provided a TSS content of 18.0 ± 1.8 mg/L, while TSS content in the case of coagulation (FeCl3) with further MF was higher 25.0 ± 3.3 mg/L. It should be noted that coagulants can reduce the sulfate content of the water; however, its examination was out of the focus of this study.
Comparison of the pretreatment methods
Comparing the flux values obtained when using 0.45 µm pore-size MF membrane, it can be stated that FeCl3 coagulant resulted in bigger flocs removed by 5–13 µm particle retention MF membrane on the one hand. By further 0.45 µm, pore-size MF membrane, 5% higher flux could be reached than by using MF alone, which is, on the other hand, not a significant deviation (Fig. 7). MF working alone could provide about 10% higher flux than PACl + MF.
Further comparison is based on chloride concentration and TSS content. After MF, the chloride concentration remained constant (Table 3), i.e., its value of 35.6 ± 0.9 mg/L was the same as the raw water's (35.9 ± 1.4 mg/L) because MF does not affect the chloride concentration. Therefore, MF is considered an environmentally benign separation process. After coagulation, chloride concentration was higher, as expected, due to the selected coagulants. Meanwhile, adding the coagulation step prior to the MF chemical addition resulted in a significant increase in the chloride concentration, ~ 6.4- and 5.7 times higher in the Cl− content in the permeate after adding FeCl3 and PACl, respectively. Both iron (Fe) and poly-aluminum (PA) are active with chloride; thus, chloride concentration will be increased in the purified water, hindering the next desalination stage. The addition of another polymer flocculent would be required. Using a further 0.45 µm pore-size MF step instead, flocs could be removed, keeping the chloride concentration of the permeate at an increased level. Therefore, the coagulation step is not preferable because it results in an elevated chloride concentration when using these two coagulants. When it is supposed to use coagulants, PACl could load the purified water with chloride in a lower amount than FeCl3; however, PACl is considered a coagulant and a flocculent at the same time.
Another comparison based on TSS content was conducted to check which process is better regarding TSS removal. Retention values were calculated based on TSS measurements according to Eq. (2):
Table 4 summarizes the TSS contents after different pretreatment steps. Regarding the average of the MF batches (0.45 µm pore-size MF membrane alone), the average value of TSS was 19.6 ± 2.5 mg/L which is remarkably lower than the initial value measured for Danube water (58 ± 1.7 mg/L). MF itself could decrease the TSS content of the raw water to 33.8% of the original value. Regarding the coagulation followed by MF, the TSS value differs because of the influence of the coagulants. Thus, choosing the best coagulant with the optimum dosage is essential to have the desired results. It is noticeable that the addition of FeCl3 provides the highest TSS content out of the three processes (25.0 ± 3.3 mg/L), which is not preferable according to our results.
Meanwhile, adding PACl with further MF results in the lowest TSS content; (18.0 ± 1.8 mg/L). From this point of view, the combined pretreatment process, i.e., coagulation with PACl + MF, is better than coagulation with FeCl3 + MF. Retention calculations show that the coagulation with PACl + MF reaches retention of 68.97%; MF alone could achieve almost the same value (66.21%). Meanwhile, FeCl3 as a coagulant shows a removal rate of 56.89% and adding a flocculant could have increased this value prior to the MF step.
The compared pretreatment methods and the measurement results are illustrated in Fig. 8.
Conclusions
During this research, the main focus was on whether microfiltration as an environmentally benign separation technique can replace the conventional coagulation pretreatment methods with high chemical consumption to prepare the appropriate make-up water treatment of thermal power plants from Danube water. It was found that microfiltration is suitable for freshwater pretreatment regarding the good quality and quantity of permeate. From an environmental point of view, according to our results, it is worth using MF alone. However, we have not done long-lasting experiments, thus having any information on fouling. Based on the findings by other researchers, severe fouling can be obtained in micro- and ultra-filtration membranes which require chemical cleaning. Using 0.45 µm pore-size microfiltration membrane alone as a pretreatment step requires no chemicals and does not influence the water's chloride concentration compared to coagulation with PACl or FeCl3. After coagulation, chloride concentration was higher, ~ 6.4- and 5.7-times more elevated in the Cl− content in the permeate after adding FeCl3 and PACl prior to MF, respectively. Both iron and poly-aluminum are active with chloride; thus, chloride concentration will be increased in the purified water, hindering the forthcoming desalination stage. MF can lower the TSS content without changing the ion content of water regarding the excellent quality and quantity of permeate, replacing conventional methods presented by coagulation–flocculation results in no chemical needs in the pretreatment stage, which increase the permeate quality. The addition of PACl with further MF resulted in the lowest TSS content (18.0 ± 1.8 mg/L). From this point of view, the combined pretreatment process, i.e., coagulation with PACl + MF, is better than FeCl3 coagulation + MF. Although coagulation with PACl + MF can decrease the TSS content of permeate to a slightly lower value than that of MF alone, the chemical addition increases the permeate's chloride concentration resulting in higher conductivity which makes the further desalination step more difficult later.
Regarding the retention value, PACl + MF was better than FeCl3 + MF in TSS removal and provided a higher retention value (around 12% higher); retention values of 68.97% and 56.89% for PACl and FeCl3, respectively. MF alone could reach the same retention value as PACl + MF (66.21%). It was illustrated that the TSS and chloride concentration are suitable parameters helping to choose the best pretreatment technology, which is cleaner, sustainable, and environmentally friendly.
References
Choshnova D (2018) Improving of the water preparation systems in the industry thermal power plants. MATEC Web Conf 145:1–7
Cote P, Masini M, Mourato D (2004) Comparison of membrane options for water reuse and reclamation. Desalination 167:1–11
Deng L, Ngo HH, Guo W, Zhang H (2019) Pre-Coagulation coupled with sponge-membrane filtration for organic matter removal and membrane fouling control during drinking water treatment. Water Res 157:155–166
Dobrin M, Tomescu CE, Ionel I, Florescu C (2012) Make-up water treatment within the water circuit of the thermal power plants. Rev Chim 63(8):839–842
Ebrahim S, Bou-Hamed S, Abdel-Jawad M, Burney N (1997) Microfiltration system as a pretreatment for RO units: technical and economic assessment. Desalination 109(2):165–175
Farahani DA, Hossein M, Borghei SM, Vatanpour V (2016) Recovery of cooling tower blowdown water for reuse: the investigation of different types of pretreatment prior nanofiltration and reverse osmosis. J Water Process Eng 10:188–199
Howe KJ, Clark MM (2006) Effect of coagulation pretreatment on membrane filtration performance. J Am Water Works Assoc 98:133–146
Jiang S, Li Y, Ladewig BP (2017) A review of reverse osmosis membrane fouling and control strategies. Sci Total Environ 595:567–583
Judd SJ, Hillis P (2001) Optimisation of combined coagulation and microfiltration for water treatment. Water Res 35(12):2895–2904
Kimura K, Tanaka K, Watanabe Y (2014) Microfiltration of different surface waters with/without coagulation: clear correlations between membrane fouling and hydrophilic biopolymers. Water Res 49:434–443
Kochkodan V, Hilal N (2015) A comprehensive review on surface modified polymer membranes for biofouling mitigation. Desalination 356:187–207
Konieczny K et al (2009) Coagulation—ultrafiltration system for river water treatment. Desalination 240(1–3):151–159
Ly QV, Nghiem LD, Cho J, Hur J (2018) Insights into the roles of recently developed coagulants as pretreatment to remove effluent organic matter for membrane fouling mitigation. J Membr Sci 564:643–652
Ma B et al (2015) Effect of aluminum speciation on ultrafiltration membrane fouling by low dose aluminum coagulation with bovine serum albumin (BSA). J Membr Sci 492:88–94
Park H, Lim S, Lee H, Woo D (2021) Water blending effects on coagulation–flocculation using aluminum sulfate (Alum), poly aluminum chloride (PAC), and ferric chloride (FeCl3) using multiple water sources. Desalin Water Treat 57:7511–7521. https://doi.org/10.1080/19443994.2015.1025583
Rajaković-Ognjanović VN, Ivojinovic DZ, Grgur BN, Rajaković LV (2011) Improvement of chemical control in the water-steam cycle of thermal power plants. Appl Therm Eng 31(1):119–128
Ravanchi T, Maryam TK, Kargari A (2009) Application of Membrane separation processes in petrochemical industry: a review. Desalination 235(1–3):199–244
Sakol D, Konieczny K (2004) Application of coagulation and conventional filtration in raw water pretreatment before microfiltration membranes. Desalination 162:61–73
Salamon E, Goda Z, Berek T (2018) Analysis of reverse osmosis filter permeability. Pollack Periodica 13(13):221–230
Schäfer AI, Fane AG, Waite TD (2001) Cost factors and chemicals pretreatment effects in the membrane filtration of waters containing natural organic matter. Water Res 35:1509–1517
Shaheen R, Cséfalvay E (2022) The role of coagulation and microfiltration in seawater pre-treatment. Period Polytech Chem Eng 66:557–564
Teng J et al (2020) Membrane fouling by alginate in poly aluminum chloride (PACl) coagulation/microfiltration process: molecular insights. Sep Purif Technol 236:116294
Wang X, Gan Y, Zhang S (2019) Improved resistance to organic matter load by compositing a cationic flocculant into the titanium xerogel coagulant. Sep Purif Technol 211:715–722
Woo SH, Min BR, Lee JS (2017) Change of surface morphology, permeate flux, surface roughness and water contact angle for membranes with similar physicochemical characteristics (except surface roughness) during microfiltration. Sep Purif Technol 187:274–284
Xu M, Luo Y, Wang X, Zhou L (2022) Coagulation-ultrafiltration efficiency of polymeric Al-, Fe-, and Ti-coagulant with or without polyacrylamide composition. Sep Purif Technol 280:119957
Acknowledgements
The project was supported by the Hungarian National Research and Innovation Office-NKFIH under Project Number FK-143215. Edit Cséfalvay is grateful for the financial support the Hungarian Academy of Sciences for the scholarship for "Gyermeket Nevelő Kutatók számára" 89/2/2022/KP. The authors thank the Department of Chemical and Environmental Process Engineering at Budapest University of Technology and Economics for using their lab facilities to accomplish these experiments. The authors thank the help of Anett Aranyosi MSc student, in the lab experiments. The research reported in this paper is part of Project No. BME-NVA-02, implemented with the support provided by the Ministry of Innovation and Technology of Hungary from the National Research, Development and Innovation Fund, financed under the TKP2021 funding scheme.
Funding
Open access funding provided by Budapest University of Technology and Economics.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Shaheen, R., Cséfalvay, E. Investigation of coagulation and microfiltration for Danube water as the first step of make-up water production. Chem. Pap. 77, 5531–5539 (2023). https://doi.org/10.1007/s11696-023-02883-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11696-023-02883-4