Abstract
This work describes the synthesis and characterization of a polymeric film of 3,5-diamino 1,2,4-triazole on a pencil graphite electrode for the selective sensing of pyridoxine (PY). The PGE was modified using the electropolymerization process by the potentiodynamic method. The polymerized electrode (PDAT/PGE) was characterized by IR, SEM, AFM, cyclic voltammetry, and electrochemical impedance spectroscopy. PY undergoes irreversible oxidation at 0.79 V on PDAT/PGE in phosphate buffer of pH 5. Using the differential pulse voltammetric technique (DPV), PY showed a linear range from 5 to 950 μM with a lower detection limit of 2.96 μM. The PDAT/PGE was applied for the analytical determination of PY in pharmaceutical tablets with good recovery.
Graphical abstract

Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Pyridoxine is a cardinal biomolecule in the human body that plays a prominent role in various cellular metabolic processes (Hellmann and Mooney 2010)(Parra et al. 2018). PY is a class of water-soluble B vitamins and is otherwise known as Vitamin B6 (Huang et al. 2021). PY also possesses significant functions in the central and peripheral nervous system (Calderón-Ospina and Nava-Mesa 2020). PY performs neuro-specific functions (Bender 1984), and the PY-related enzymes are actively involved in the synthesis of different neurotransmitters (Dakshinamurti et al. 1990) (Rejithamol and Beena 2020). Pyridoxal 5′-phosphate (PLP) is the active form of vitamin B6, and the PY concentration in blood plasma is usually measured in terms of PLP (Information 2015). The marginal values of PLP in plasma are 20–30 nM, and thus PY deficiency is associated with plasma PLP values of < 20 nM (Jungert et al. 2020). Several diseases, such as cardiovascular diseases, gastric disorders, diabetes, neurological disorders, immune deficiency, and cancer, are associated with low concentrations of vitamin B6 in plasma (Kim and Cho 2014)(Merigliano et al. 2018)(Kjeldby et al. 2013). According to literature reports, PY possesses anti-inflammatory properties, and thus supplementation of vitamin B6 can be used to alleviate viral infections(Santhy et al. 2020)(Kumrungsee et al. 2020)(Zhang et al. 2016). Recent studies reported that supplementation of PY significantly relieves the worse health conditions associated with Covid-19 patients, such as cardiovascular diseases, diabetes, hypertension, etc. (Huang et al. 2021)(Stach et al. 2021). PY is essential for maintaining healthy skin, and its deficiency may cause dermatitis (Kato 2012). Hence vitamin B6 has been used as a supplement to treat dermatitis and other skin diseases(Wright et al. 1943)(Mabin et al. 1995). Various cases of neuropathies have been reported in patients due to the excessive intake of vitamin B6 supplements (Gdynia et al. 2008; van Hunsel et al. 2018; Vrolijk et al. 2020). The European food safety authority has recommended lowering the upper-level dosage of vitamin B6 intake from 100 to 25 mg/day (Vrolijk et al. 2017). Hence the usage of the pharmaceutical supplements of PY has received more attention in the last few years.
From the analytical point of view, it is of utmost importance to develop highly efficient and accurate sensors to determine the PY concentrations from biological and pharmaceutical samples. Since more advanced scientific methods are used in clinical and pharmaceutical industries, the demand for developing disposable and portable devices is increasing. According to literature reports, multiple techniques have been employed to determine PY, including spectrophotometry, chromatography, spectrofluorimetry, etc. (Gatti and Gioia 2005; Sorouraddin et al. 2005). But these analytical techniques are more expensive, require lengthy procedures, and could be considered as the limitations (Hadi Beitollahi and Fariba Garkani Nejad 2019). However, compared to all the analytical techniques mentioned above, electrochemical methods have more advantages as it offers easy operating procedure, fast response, and a cost-effective detection platform for various analytes (Sharma and Arya 2019). Hence splendid research is going on for the development and application of sensing devices based on electrochemical methods in environmental, food, pharmaceutical, clinical fields, etc.(Antherjanam and Saraswathyamma 2021; Krishnan and Saraswathyamma 2021; Li et al. 2015; Madrakian et al. 2014).
In electrochemical detection methodologies, PGEs have attracted more attention than other carbon-based electrodes and are widely utilized in many electroanalytical applications (David et al. 2017). PGEs are one of the highly economical electrodes which possess various characteristics suitable for electrochemical sensor fabrication (Kolahi-Ahari et al. 2020). The easy preparation methods, high sensitivity, and low background current highlight its demand in the area of electrochemical sensors (Sankaranarayanan and Venkateswaran 2020) (Ganesh et al. 2018). Chemically modified electrodes are used to increase the sensitivity and selectivity of the electrochemical sensors (Zuo et al. 2012). The applications of electropolymers as modifiers in the fabrication of electroanalytical devices are reported in previous literature (Sajid et al. 2015) (Muti et al. 2013a). The thickness and morphology of the polymers can be carefully controlled using electropolymerization techniques, and polymerization can be done by in situ methods by a simple procedure (Imisides et al. 1991) (Vedhi et al. 2009). Also, the polymers obtained via electropolymerization techniques were highly pure, and even these polymers can be prepared in an aqueous solution, which is not possible in chemical polymerization methods (Gvozdenović et al. 2014). Hence the use of electropolymers is of great interest in modifying various electrode surfaces in electrochemical applications. Conducting as well as insulating polymers can be generated using electropolymerization by potentiodynamic method, and their applications in the surface modification are also reported (Yuqing et al. 2004) (Gvozdenović et al. 2014) (Bonyadi and Ghanbari 2021). Conducting polymers are one of the attractive materials used for the modification of various electrodes in electrochemical sensor developments (Lee et al. 2012; Meng et al. 2021). Conducting polymers offers more stability and excellent electrical properties that extend its applicability in the fabrication of electrochemical sensors(Chen et al. 2013; Nie et al. 2013). The organic compounds with heterocyclic rings and nitrogen-containing aromatic compounds are promising scaffolds for synthesizing conducting polymers (Waltman and Bargon 1986; Yadav et al. 2013). Hence triazoles and triazole-derived compounds are appropriate materials for the generation of conducting polymers, and it has been proven in literature reports (Danyıldız et al. 2017; Revin and John 2012).
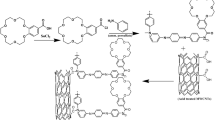
In this report, we have introduced a conducting polymer of 3,5-diamino 1,2,4-triazole (DAT) for the electrochemical determination of PY in pharmaceutical samples. PGE was used as the electrode substrate, and the PDAT film was generated on the PGE surface by cyclic voltammetry. DAT has been previously used to fabricate electrochemical sensors for pharmaceuticals, biomolecules, environmental pollutants, etc. (Calam 2021; Kesavan and Abraham John 2014; Kumar et al. 2017). The mechanism of electropolymerization was investigated by ATR-IR spectroscopy and its morphological studies were done by SEM and AFM techniques.
Moreover, electrochemical studies were done by cyclic voltammetry (CV) and electrochemical impedance spectroscopy (EIS) analysis. The obtained PDAT/PGE sensors showed high sensitivity and stability towards the electrooxidation of PY. In addition, this is the first work reported for the electropolymerization of DAT on PGE, according to our knowledge. The results suggested that the PDAT/PGE might be a reliable sensor for the sensitive determination of PY in pharmaceutical formulations.
Experimental
Reagents and materials
DAT, monosodium dihydrogen phosphate (NaH2PO4), and disodium orthohydrogen phosphate (Na2HPO4) were obtained from Merck. PY was purchased from Loba Chemie. Analytical grade chemical reagents were utilized for preparing all solutions and all solutions were made up in Millipore water. PGE of 0.7 mm diameter manufactured by Cello writing Aids Pvt. Ltd. was procured from a local supermarket. PY tablets containing 100 mg per tablet were purchased from a pharmaceutical shop.
Instrumentation
The electrochemical analyzer of model CHI 610E, CH Instruments, USA, was used for all voltammetric studies. The experimental setup consists of a three-electrode system with Pt wire as the counter electrode and Ag–AgCl (1 M KCl) electrode as the reference electrode. The modified electrode (PDAT/PGE) was used as the working electrode. These electrodes were placed in an electrochemical cell containing 10 mL supporting electrolyte for the electrochemical studies. Scanning electron microscopic images (SEM) were obtained using the JEOL model: JSM 6490La. ATR-IR analysis of the electrodes was done using Perkin Elmer Spectrum Two IR Spectrometer. The ATR-IR analysis was performed in a range of 500–4500 cm−1. EIS analyses were performed using the Autolab bi-potentiostat PGSTAT 302N instrument. A potential amplitude of 0.1 V in a frequency range of 0.1–1000 Hz was used for the EIS analysis. AFM analysis in tapping mode was done using Bruker Dimension Edge atomic force microscope.
Electropolymerization of DAT
The electropolymerization of DAT was carried out in an electrochemical cell containing three electrode systems with PGE as the working electrode, Ag–AgCl (1 M KCl) as the reference electrode, and Pt wire as the counter electrode. PGE was covered by a teflon tape exposing a length of 5 mm, and the uncovered portion was used for the polymerization process. The opposite side of the exposed part of PGE is used for electrical contact. PDAT film was generated on PGE using CV in a monomer solution containing 15 mM DAT dissolved in 0.1 M KCl. Chloride ion containing electrolytes have been used in various electropolymerization process according to the literature reports (S. Chen et al. 2018; Zembrzuska et al. 2019). The CV was done in a potential range of − 0.8 to 1.6 V at a scan rate of 100 mV/s. After ten cycles, the obtained PDAT/PGE was thoroughly washed with millipore water and used for further studies.
Electroanalytical measurements
CV and DPV were used for the study of electrochemical oxidation and quantification of PY on PDAT/PGE. CV was performed by sweeping the potential from 0.5 V to 1.2 V with a scan rate of 100 mV/s. DPV measurements were also done in the potential window of 0.5 to 1.2 V. The CV and DPV measurements were performed by adding a known volume of PY to 10 mL 0.1 M PBS pH 5 contained in an electrochemical cell.
Results and discussions
Morphological studies of PDAT/PGE
Figure 1A and 1B display the SEM images of PGE and PDAT/PGE. The graphite flakes are noticeable on the surface of PGE, whereas flake-like morphology is not there in the case of polymer-coated PGE. This indicated the successful polymerization of DAT on the bare graphite surface. The 2D and 3D AFM images of unmodified PGE and PDAT/PGE are given in Fig. 2. From the AFM data obtained, Rq of the PGE and PDAT/PGE were 0.0536 µm and 0.0625 µm respectively, where Rq is the root mean square roughness value. The increase in Rq value of PDAT/PGE indicates the formation of a polymeric film on pencil graphite surface after the modification process.
Electrochemical characterization of PDAT film
The PDAT/PGE and PGE were electrochemically studied by the fundamental electroanalytical technique, CV and EIS using 5 × 10–3 M potassium ferricyanide in 0.1 M KCl as the redox probe. CV images are displayed in Fig. 3A, and the electrochemical behavior of the redox probe was observed on both electrodes. The current response of redox peaks gets increased in the case of PDAT/PGE than PGE in the CV measurements. Thus, the polymer film on the PGE is more conducting than the bare graphite surface; consequently, the modified PGE enhances the electron transfer rate of the redox reaction. EIS is a fundamental electroanalytical technique used to study the electrochemical behavior of the electrode surface (Sharma et al. 2020). Figure 3B represents the Nyquist plot for the PGE and PDAT/PGE in 5 × 10–3 M potassium ferricyanide in 0.1 M KCl solution. The Randles circuit for the electrodes is given in the inset of Fig. 3B and R1 is the resistance of the electrolyte solution, R2 represents the charge transfer resistance of the electrode, Q is the constant phase element and W is the Warburg impedance(Antherjanam and Saraswathyamma 2022). The Warburg component denotes the diffusion of ion the electrochemical oxidation reactions and the R1 is independent on the frequency (Balasubramani et al. 2020; Randviir and Banks 2013). The Nyquist plot with Randles circuit fitted data is given in supplementary figure S1. The semicircle in the higher frequency region of the Nyquist plot represents the charge transfer limited process (Shishkanova and Sinica 2022). From Fig. 3B, PGE represents the largest diameter, and PDAT/ PGE showed the smallest diameter for the semicircle. Hence it is concluded that the polymeric film of DAT on PGE is more conducting than bare PGE.
A CV of bare PGE and PDAT/PGE in 5 mM ferricyanide in 0.1 M KCl at 100 mV/s B Nyquist plot of bare PGE and PDAT/PGE in 5 mM ferricyanide in 0.1 M KCl and corresponding Randles circuit is given in inset C CV of electropolymerization of DAT on PGE D CV of PY on PGE and PDAT/PGE at 100 mV/s in PBS pH 5
ATR-IR analysis of DAT, PGE, and PDAT/PGE
The formation of PDAT film on PGE can be explained using the ATR-IR spectra of DAT, PDAT/PGE, and bare PGE. The IR spectrum of the monomer DAT is given in figure S2A. DAT showed peaks at 1578 cm−1 and 1068 cm−1 corresponding to C=N and N–N stretching frequencies. However, the peaks due to C=N and N–N stretching are slightly shifted to 1651 cm−1 and 1046 cm−1, respectively (Tabanlıgil Calam 2019). The band due to C–N stretching is observed at 1143 cm−1 on DAT and 1151 cm−1 on PDAT/PGE (Calam 2020). C–H stretching bands are observed at 3107 cm−1 and 2989 cm−1 on DAT and PDAT/PGE, and the peaks are in good agreement with the reported literature (Calam 2020). IR bands in the range of 3300 cm−1 are observed on DAT, indicating the presence of the aromatic –NH2 group in the monomer (Ismail et al. 2015). But on comparison of the IR spectra of PGE and PDAT/PGE (Figure S2B and S2C), no new peaks were observed on PDAT/PGE corresponding to the –N–H stretching of primary aromatic amine. This justified the involvement of the NH2 group in the polymerization process, as reported in the previous literature. Further, a new peak is obtained on PDAT/PGE at 1439 cm−1, which corresponds to N=N stretching, and this peak is not visible in the spectrum of DAT (Aydoǧdu et al. 2014; Muti et al. 2013b; Revin and John 2011). The presence of N=N stretching in the modified electrode successfully proved the generation of PDAT film on the PGE.
Mechanism of formation of PDAT film on PGE
The CV of electropolymerization of DAT (Fig. 3C) showed strong oxidation peak at 0.49 V and cathodic peaks at 0.51 V, 0.18 V, and − 0.11 V. These peak potentials are observed to be slightly shifted from the reported polymerization of DAT on bare GCE (Kumar et al. 2017). Following the reported mechanism in (Kesavan and Abraham John 2014; Kumar et al. 2017), the anodic peak at 0.49 V corresponds to the oxidation of the aromatic primary NH2 group to form a radical cation (Antherjanam and Saraswathyamma 2021). Then the radical cations undergo polymerization through the –NH–NH– linkage. At 0.74 V, the –NH–NH– further undergo dimerization to form another radical cation. Then the unstable radical cations get dimerized to form PDAT film on the PGE. According to the reported mechanism, the plausible mechanism of electro generation of DAT film on PGE is given in figure S3.
Electrochemistry of PY on PDAT/PGE and PGE
Figure 3D represents the CV of 50 μM PY on PGE and PDAT/PGE in PBS pH 5 in a potential scanning from 0.5 to 1.2 V at 100 mV/s. An oxidation peak for PY was obtained on the bare PGE and PDAT/PGE at 0.83 V. However, the current response of PY was increased on PDAT/PGE than on the bare PGE surface. According to previous literature, the electrooxidation of PY occurs through a two proton-two electron transfer (Cherian et al. 2021). Thus, the alcoholic group opposite the nitrogen in the pyridine ring is oxidized to the aldehyde group, as this hydroxyl group is highly reactive compared to other hydroxyl groups present in the ring structure of PY (Rejithamol and Beena 2020). The suggested mechanism of electrooxidation of PY is given in supplementary figure S4.
Optimization studies
Supporting electrolyte
As DPV is more sensitive than CV, analytical quantification of PY was done using the DPV method (Vadivaambigai et al. 2015). Among the different supporting electrolytes studied (0.1 M KCl, 0.1 M H2SO4, 0.1 M acetate buffer pH 5.6, 0.1 M PBS pH 5), a better current response for 25 μM PY was obtained on PDAT/PGE in PBS pH 5(Data given in supplementary table T1). The pH of the PBS was also optimized from pH 4 to 8 (supplementary figure S5 A), and a better current response was obtained for PBS pH 5. Hence all further analytical studies were optimized with PBS pH 5. The oxidation peak current increased from pH 4 to 5 and then gradually decreased with a further increase in pH. This showed the involvement of the pH of the electrolyte in the electrooxidation of PY on PDAT/PGE. Thus, at higher pH, the deprotonation of PY will occur, slowing down the oxidation process, and therefore peak current decreases (Cherian et al. 2021). The influence of pH in the electrooxidation of PY was corroborated by the negative shift of peak potentials with the increase in pH (figure S6) (Zhang et al. 2017). Also, the plot of pH of the supporting electrolyte against peak potentials was linear with the following linear regression equation.
Epa = 1.1164 + − 0.06192 pH, R2 = 0.99861
Thus, the slope obtained of 61.92 mV/pH was almost equal to the Nernstian slope. Therefore, an equal number of protons and electrons are involved in the electrooxidation of PY (Filik et al. 2017). Hence the mechanism mentioned above of the electrochemical oxidation of PY on PDAT/PGE is validated.
Further, the effect of DAT concentration on the electrooxidation of PY was also examined (5–20 mM). The oxidation peak with more peak current was obtained for 15 mM DAT concentration (result displayed in supplementary S5 B). After all, the polymerization cycles were also varied from 5 to20 cycles to find out the best result for the electrochemical oxidation of PY. The polymerization cycle was then finally selected as ten cycles, providing the best outcome for the electroanalysis of PY (supplementary Figure S5C).
Scan rate study
CV was used to examine the dependence of scan rate on the oxidation potential of PY. Figure 4A shows the cyclic voltammogram of 50 μM PY in 0.1 M PBS of pH 5 from varying scan rates of 10–120 mV/s. The voltammogram indicates that the oxidation current of PY is increased with an increasing scan rate. Also, the peak potentials are shifted to more positive values as the scan rate increases, which is attributed to the irreversible nature of the oxidation process (Sartori et al. 2010). The peak currents of PY against the square root of scan rate (v1/2) were plotted (Fig. 4B), and they showed good linearity, implying a diffusion-controlled nature of the electrooxidation process (Stanković and Kalcher 2015). In addition, the logarithm of peak current showed linear dependence with the logarithm of scan rate with a slope of 0.445 (Figure S7A). This validated the diffusion-controlled electrochemical reaction of PY on PDAT/PGE, as the slope obtained is almost equal to the theoretical value of 0.5 (Deepa G. Patil, Naveen M. Gokavi and Nandibewoor, 2016). The total number of electrons (n) participated in the electrochemical oxidation of PY was calculated using the equation, Epa = (2.303RT/αnF) log υ + constant where α is the electron transfer rate constant and is assumed to be 0.5 for an irreversible electrode reaction (Figure S7B) (Oliveira et al. 2013). The other terms involved in the equations are Epa, the oxidation peak potential (V), F, the Faraday’s constant (96,485 C/mol), R, the gas constant (8.314 J /K mol), and T, the temperature (K). The number of calculated electrons was 2.36, which is approximately equal to 2.
Electroanalysis of pyridoxine on the modified electrode
DPV profiles of PY in the concentration range of 5–950 μM in 0.1 M PBS (pH 5) using the PDAT/PGE is displayed in Fig. 5A. As seen from the figure, the oxidation peak currents of PY increase successively with the concentration. Figure 5B shows the calibration curve of different concentrations of PY obtained from the oxidation peak currents. The anodic peak current increased linearly with two slopes ranging from 5–450 μM and 450–950 μM, respectively, with a LOD of 2.96 μM at a signal-to-noise ratio of 3.
Interference studies
Selectivity is an important parameter to be studied in the development of electrochemical sensors, as this could identify the interfering species that may influence the analytical determination of analytes. DPVs of the PDAT/PGE in 0.1 M PBS pH 5 containing 50 μM PY with addition of NaCl (5 mM), KCl (5 mM), trisodium citrate (Na3C6H5O7) (0.1 mM), MgSO4 (0.1 mM), riboflavin (100 μM), folic acid (80 μM), ascorbic acid (80 μM), CaCl2 (80 μM), FeCl3(80 μM), glucose (1.25 mM), and urea (5 mM) is presented in Fig. S8. The modified electrode showed a signal change of only less than 10% for the quantification of PY in the presence of excess amounts of the species mentioned above. Thus, the PDAT/PGE is selective for the electrochemical analysis of PY in real samples.
Reproducibility and stability studies
The reproducibility of the electrodes towards the electrooxidation of PY was measured from the DPV response of three independently fabricated electrochemical sensors in 0.1 M PBS pH 5. The RSD of the three electrodes’ electrochemical response was calculated and was about 5.93%. Also, three different PDAT/PGE was kept in water in the refrigerator for 15 days, and the stability of the electrode was studied in 0.1 M PBS pH 5 containing 50 μM PY. The electrodes showed excellent stability with an RSD of 5.3%. Hence the modified electrode was proved to be highly efficient in developing an electrochemical device for the real-time monitoring of PY. The analytical performance of the PDAT/PGE and other recently reported electrochemical sensors for PY for the pharmaceutical application has been listed in Table 1 for comparison. Thus, the analytical performance of PDAT/PGE towards the electrochemical quantification of PY in pharmaceutical samples is better than the reported sensors in Table 1.
Real sample analysis
The PDAT/PGE was applied for the analytical quantification of PY from commercially available vitamin B6 tablets using DPV at optimized conditions. The PY tablets were made into a fine powder, and a 5 mM tablet solution was prepared by transferring a suitable amount of powdered tablet into 10 mL of deionized water. Then two different concentrations of the tablet solutions were spiked into PBS pH 5, and the recoveries of the tablet from the spiked solutions were estimated (Table 2).
Conclusion
Pencil graphite electrode, PGE modified with poly 3,5-diamino 1,2,4- triazole (PDAT) were prepared and used for the electrochemical determination of pyridoxine, PY in phosphate buffer solution. Electropolymerization technique was employed for the modification process and to our knowledge this is the first work reporting the electropolymerizion of DAT on PGE surface. Cyclic voltammetry and differential pulse voltammetry were used for the analytical determination of PY. The developed sensor has advantages such simple fabrication procedure, cost effectiveness, wide linear range, good selectivity, etc. for the electrochemical quantification of PY. The remarkable stability and reproducibility make the proposed method a promising tool for the determination of PY in pharmaceutical samples. Moreover, the electrode was also favourably employed in the monitoring of PY in pharmaceutical samples with acceptable range of recoveries.
References
Amini R, Asadpour-Zeynali K (2020) Cauliflower-like NiCo2O4−Zn/Al layered double hydroxide nanocomposite as an efficient electrochemical sensing platform for selective pyridoxine detection. Electroanalysis 32(6):1160–1169. https://doi.org/10.1002/elan.201900600
Antherjanam S, Saraswathyamma B (2021) Simultaneous electrochemical determination of hydrazine and hydroxylamine on a thiadiazole derivative modified pencil graphite electrode. Mater Chem Phys 275:125223. https://doi.org/10.1016/j.matchemphys.2021.125223
Antherjanam S, Saraswathyamma B (2022) Simultaneous electroanalysis of adenine and adenosine on a poly-nicotinic acid modified pencil graphite electrode. Mon Fur Chem 153(12):1143–1153. https://doi.org/10.1007/s00706-022-02985-x
Apetrei IM, Apetrei C, Buzia OD (2016) Ordered mesoporous carbon based sensor for sensitive detection of vitamin B6 in pharmaceuticals. Farmacia 64(4):544–548
Aydoǧdu G, Günendi G, Zeybek DK, Zeybek B, Pekyardimci Ş (2014) A novel electrochemical DNA biosensor based on poly-(5-amino-2-mercapto-1, 3,4-thiadiazole) modified glassy carbon electrode for the determination of nitrofurantoin. Sens Actuators B Chem 197:211–219. https://doi.org/10.1016/j.snb.2014.02.083
Balasubramani V, Chandraleka S, Rao TS, Sasikumar R, Kuppusamy MR, Sridhar TM (2020) Review—recent advances in electrochemical impedance spectroscopy based toxic gas sensors using semiconducting metal oxides. J Electrochem Soc. https://doi.org/10.1149/1945-7111/ab77a0
Beitollahi H, Nejad FG (2019) A carbon paste electrode modified by graphene oxide/Fe3o4@Sio2/ionic liquid nanocomposite for voltammetric determination of acetaminophen in the presence of tyrosine. Russ J Electrochem 55(12):1162–1170. https://doi.org/10.1134/S1023193519120024
Bender D (1984) B vitamins in the nervous system. Neurochem Int 6(3):297–321. https://doi.org/10.1016/0197-0186(84)90072-X
Bonyadi S, Ghanbari K (2021) Electrochemical synthesis of poly(melamine)-poly(aspartic acid) copolymer for highly sensitive and selective determination of dopamine. Mater Chem Phys 267:124683. https://doi.org/10.1016/j.matchemphys.2021.124683
Calam TT (2020) electrochemical oxidative determination and electrochemical behavior of 4-nitrophenol based on an au electrode modified with electro-polymerized 3,5-diamino-1,2,4-triazole film. Electroanalysis 32(1):149–158. https://doi.org/10.1002/elan.201900450
Calam TT (2021) Selective and sensitive determination of paracetamol and levodopa with using electropolymerized 3,5-diamino-1,2,4-triazole film on glassy carbon electrode. Electroanalysis. https://doi.org/10.1002/elan.202060477
Calderón-Ospina CA, Nava-Mesa MO (2020) B Vitamins in the nervous system: current knowledge of the biochemical modes of action and synergies of thiamine, pyridoxine, and cobalamin. CNS Neurosci Ther 26(1):5–13. https://doi.org/10.1111/cns.13207
Chen L, Li K, Zhu H, Meng L, Chen J, Li M, Zhu Z (2013) A chiral electrochemical sensor for propranolol based on multi-walled carbon nanotubes/ionic liquids nanocomposite. Talanta 105:250–254. https://doi.org/10.1016/j.talanta.2012.12.035
Chen S, Liu S, Wen A, Zhang J, Nie H, Chen J, Zeng R, Long Y, Jin Y, Mai R (2018) New insight into electropolymerization of melamine. I: chloride promoted growth of polymelamine in different pH medium. Electrochim Acta 271:312–318. https://doi.org/10.1016/j.electacta.2018.03.146
Cherian AR, Benny L, Varghese A, John NS, Hegde G (2021) Molecularly imprinted scaffold based on poly(3-aminobenzoic acid) for electrochemical sensing of vitamin B6. J Electrochem Soc 168(7):077512. https://doi.org/10.1149/1945-7111/ac1494
Dakshinamurti K, Paulose CS, Viswanathan M, Siow YL, Sharma SK, Bolster B (1990) Neurobiology of pyridoxine. Ann NY Acad Sci 585(1):128–144. https://doi.org/10.1111/j.1749-6632.1990.tb28049.x
Danyıldız Z, Uzun D, Calam TT, Hasdemir E (2017) A voltammetric sensor based on glassy carbon electrode modified with 1H–1,2,4-triazole-3-thiol coating for rapid determination of trace lead ions in acetate buffer solution. J Electroanal Chem. https://doi.org/10.1016/j.jelechem.2017.09.042
David IG, Florea MA, Cracea OG, Popa DE, Buleandra M, Iorgulescu EE, David V, Badea IA, Ciucu AA (2015) Voltammetric determination of B1 and B6 vitamins using a pencil graphite electrode. Chem Pap 69(7):901–910. https://doi.org/10.1515/chempap-2015-0096
David IG, Popa DE, Buleandra M (2017) Pencil graphite electrodes: a versatile tool in electroanalysis. J Anal Methods Chem. https://doi.org/10.1155/2017/1905968
Filik H, Avan AA, Mümin Y (2017) Simultaneous electrochemical determination of caffeine and vanillin by using poly(alizarin red s) modified glassy carbon electrode. Food Anal Methods 10(1):31–40. https://doi.org/10.1007/s12161-016-0545-z
Ganesh PS, Kumara Swamy BE, Fayemi OE, Sherif ESM, Ebenso EE (2018) Poly(crystal violet) modified pencil graphite electrode sensor for the electroanalysis of catechol in the presence of hydroquinone. Sens Bio-Sens Res 20:47–54. https://doi.org/10.1016/j.sbsr.2018.08.001
Gatti R, Gioia MG (2005) Liquid chromatographic determination with fluorescence detection of B 6 vitamers and riboflavin in milk and pharmaceuticals. Anal Chim Acta 538(1–2):135–141. https://doi.org/10.1016/j.aca.2005.02.025
Gdynia HJ, Müller T, Sperfeld AD, Kühnlein P, Otto M, Kassubek J, Ludolph AC (2008) Severe sensorimotor neuropathy after intake of highest dosages of vitamin B6. Neuromuscul Disord. https://doi.org/10.1016/j.nmd.2007.09.009
Gvozdenović MM, Jugović BZ, Stevanović JS, Grgur BN (2014) Electrochemical synthesis of electroconducting polymers. Hem Ind 68(6):673–684. https://doi.org/10.2298/HEMIND131122008G
Hellmann H, Mooney S (2010) Vitamin B6: a molecule for human health? Molecules 15(1):442–459. https://doi.org/10.3390/molecules15010442
Huang L, Tian S, Zhao W, Liu K, Guo J (2021) Electrochemical vitamin sensors: a critical review. Talanta 222:121645. https://doi.org/10.1016/j.talanta.2020.121645
Imisides MD, John R, Riley PJ, Wallace GG (1991) The use of electropolymerization to produce new sensing surfaces: a review emphasizing electrode position of heteroaromatic compounds. Electroanalysis 3(9):879–889. https://doi.org/10.1002/elan.1140030903
Information N (2015). Vitamin B61. https://doi.org/10.3945/an.113.005207.TABLE
Ismail H, Mirza B, Haq I, Shabbir M, Akhter Z, Basharat A (2015) Synthesis, characterization and pharmacological evaluation of selected aromatic amines 2015
Jungert A, Linseisen J, Wagner KH, Richter M (2020) Revised D-A-CH reference values for the intake of vitamin B6. Ann Nutr Metab 76(4):213–222. https://doi.org/10.1159/000508618
Kato N (2012) Role of vitamin B6 in skin health and diseases. https://doi.org/10.3920/978-90-8686-729-5_4
Kesavan S, Abraham John S (2014) Fabrication of aminotriazole grafted gold nanoparticles films on glassy carbon electrode and its application towards the simultaneous determination of theophylline and uric acid. Sens Actuators B Chem 205:352–362. https://doi.org/10.1016/j.snb.2014.08.086
Kim YN, Cho YO (2014) Evaluation of vitamin B6 intake and status of 20- to 64-year-old koreans. Nurs Res Pract 8(6):688–694. https://doi.org/10.4162/nrp.2014.8.6.688
Kjeldby IK, Fosnes GS, Ligaarden SC, Farup PG (2013) Vitamin B6 deficiency and diseases in elderly people-a study in nursing homes. BMC Geriatr 13(1):1–8. https://doi.org/10.1186/1471-2318-13-13
Kolahi-Ahari S, Deiminiat B, Rounaghi GH (2020) Modification of a pencil graphite electrode with multiwalled carbon nanotubes capped gold nanoparticles for electrochemical determination of tramadol. J Electroanal Chem 862:113996. https://doi.org/10.1016/j.jelechem.2020.113996
Krishnan RG, Saraswathyamma B (2021) Disposable electrochemical sensor for coumarin induced milk toxicity in raw milk samples. Mea J Int Meas Confed 170:108709. https://doi.org/10.1016/j.measurement.2020.108709
Kumar DR, Manoj D, Santhanalakshmi J, Shim JJ (2015) Au-CuO core-shell nanoparticles design and development for the selective determination of Vitamin B6. Electrochim Acta 176:514–522. https://doi.org/10.1016/j.electacta.2015.07.034
Kumar DR, Kesavan S, Baynosa ML, Shim JJ (2017) 3,5-Diamino-1,2,4-triazole@electrochemically reduced graphene oxide film modified electrode for the electrochemical determination of 4-nitrophenol. Electrochim Acta 246:1131–1140. https://doi.org/10.1016/j.electacta.2017.06.116
Kumrungsee T, Zhang P, Chartkul M, Yanaka N (2020) Potential role of vitamin B6 in ameliorating the severity of COVID-19 and its complications. Front Nutr 7:1–5. https://doi.org/10.3389/fnut.2020.562051
Kuzmanović D, Khan M, Mehmeti E, Nazir R, Amaizah NRR, Stanković DM (2016) Determination of pyridoxine (Vitamin B6) in pharmaceuticals and urine samples using unmodified boron-doped diamond electrode. Diam Relat Mater 64:184–189. https://doi.org/10.1016/j.diamond.2016.02.018
Lee E, Ahmed MS, You JM, Kim SK, Jeon S (2012) Conducting polymer-coated, palladium-functionalized multi-walled carbon nanotubes for the electrochemical sensing of hydroxylamine. Thin Solid Films 520(21):6664–6668. https://doi.org/10.1016/j.tsf.2012.07.019
Li B, Zhou Y, Wu W, Liu M, Mei S, Zhou Y, Jing T (2015) Highly selective and sensitive determination of dopamine by the novel molecularly imprinted poly(nicotinamide)/CuO nanoparticles modified electrode. Biosens Bioelectron 67:121–128. https://doi.org/10.1016/j.bios.2014.07.053
Liu SQ, Sun WH, Li LC, Li H, Wang XL (2012) Electrocatalytic oxidation and voltammetric determination of vitamin B6 by a ssDNA-modified electrode. Int J Electrochem Sci 7(1):324–337
Mabin DC, Hollis S, Lockwood J, David TJ (1995) Pyridoxine in atopic dermatitis. Br J Dermatol 133(5):764–767. https://doi.org/10.1111/j.1365-2133.1995.tb02752.x
Madrakian T, Soleimani M, Afkhami A (2014) Simultaneous determination of mycophenolate mofetil and its active metabolite, mycophenolic acid, by differential pulse voltammetry using multi-walled carbon nanotubes modified glassy carbon electrode. Mater Sci Eng C 42:38–45. https://doi.org/10.1016/j.msec.2014.05.012
Manoj D, Rajendran S, Qin J, Sundaravadivel E, Yola ML, Atar N, Gracia F, Boukherroub R, Gracia-Pinilla MA, Gupta VK (2019) Heterostructures of mesoporous TiO 2 and SnO 2 nanocatalyst for improved electrochemical oxidation ability of vitamin B6 in pharmaceutical tablets. J Colloid Interface Sci 542:45–53. https://doi.org/10.1016/j.jcis.2019.01.118
Meng L, Turner APF, Mak WC (2021) Conducting polymer-reinforced laser-irradiated graphene as a heterostructured 3D transducer for flexible skin patch biosensors. ACS Appl Mater Interfaces. https://doi.org/10.1021/acsami.1c13164
Merigliano C, Mascolo E, Burla R, Saggio I, Vernì F (2018) The relationship between vitamin B6 diabetes and cancer. Front Genet 9:1–5. https://doi.org/10.3389/fgene.2018.00388
Motaghedifard M, Behpour M, Ghoreishi SM, Honarmand E (2016) Electro-deposition of gold nanostructures on carbon paste electrode: a platform with signal amplification for voltammetric study and determination of pyridoxine (vitamin B6). Russ J Electrochem 52(5):477–487. https://doi.org/10.1134/S1023193516050098
Muti M, Gençdaĝ K, Nacak FM, Aslan A (2013) Electrochemical polymerized 5-amino-2-mercapto-1,3,4-thiadiazole modified single use sensors for detection of quercetin. Colloids Surf B 106:181–186. https://doi.org/10.1016/j.colsurfb.2013.01.018
Nejad FG, Beitollahi H, Tajik S, Jahani S (2019) La3+-doped Co3O4 nanoflowers modified graphite screen printed electrode for electrochemical Sensing of vitamin B6. Anal Bioanal Chem Res. https://doi.org/10.22036/abcr.2018.134444.1212
Nie T, Xu JK, Lu LM, Zhang KX, Bai L, Wen YP (2013) Electroactive species-doped poly(3,4-ethylenedioxythiophene) films: enhanced sensitivity for electrochemical simultaneous determination of vitamins B2, B6 and C. Biosens Bioelectron 50:244–250. https://doi.org/10.1016/j.bios.2013.06.057
Oliveira GG, Azzi DC, Vicentini FC, Sartori ER, Fatibello-Filho O (2013) Voltammetric determination of verapamil and propranolol using a glassy carbon electrode modified with functionalized multiwalled carbon nanotubes within a poly (allylamine hydrochloride) film. J Electroanal Chem 708:73–79. https://doi.org/10.1016/j.jelechem.2013.09.016
Parra M, Stahl S, Hellmann H (2018) Vitamin B6 and its role in cell metabolism and physiology. Cells. https://doi.org/10.3390/cells7070084
Patil DG, Naveen M, Gokavi AMB, Nandibewoor ST (2016) Electrochemical characterization and determination of tramadol drug using graphite pencil electrode. Anal Bioanal Electrochem 8(1):78–91
Pereira DF, Santana ER, Spinelli A (2022) Electrochemical paper-based analytical devices containing magnetite nanoparticles for the determination of vitamins B2 and B6. Microchem J 179:107588. https://doi.org/10.1016/j.microc.2022.107588
Porada R, Fendrych K, Baś B (2021) Electrochemical sensor based on Ni-exchanged natural zeolite/carbon black hybrid nanocomposite for determination of vitamin B6. Microchim Acta. https://doi.org/10.1007/s00604-021-04992-x
Randviir EP, Banks CE (2013) Electrochemical impedance spectroscopy: an overview of bioanalytical applications. Anal Methods. https://doi.org/10.1039/c3ay26476a
Rejithamol R, Beena S (2020) Electrochemical quantification of pyridoxine (VB6) in human blood from other water-soluble vitamins. Chem Pap 74(6):2011–2020. https://doi.org/10.1007/s11696-019-01049-5
Revin SB, John SA (2011) Electropolymerization of 3-amino-5-mercapto-1,2,4-triazole on glassy carbon electrode and its electrocatalytic activity towards uric acid. Electrochim Acta 56(24):8934–8940. https://doi.org/10.1016/j.electacta.2011.07.125
Revin SB, John SA (2012) Selective and sensitive electrochemical sensor for L-methionine at physiological ph using functionalized triazole polymer film modified electrode. Electroanalysis. https://doi.org/10.1002/elan.201200106
Sajid M, Nazal MK, Mansha M, Alsharaa A, Muhammad S, Jillani S, Basheer C (2015) Chemically modified electrodes for electrochemical detection of dopamine in presence of uric acid and ascorbic acid : a review trends in analytical chemistry chemically modified electrodes for electrochemical detection of dopamine in the presence of uric. Trends Anal Chem 76:15–29. https://doi.org/10.1016/j.trac.2015.09.006
Sankaranarayanan P, Venkateswaran SM (2020) Affordable voltammetric sensor based on anodized disposable pencil graphite electrodes for sensitive determination of dopamine and uric acid in presence of high concentration of ascorbic acid. J Electrochem Sci Eng 10(3):263–279. https://doi.org/10.5599/jese.783
Santhy A, Saraswathyamma B, Parvathy Krishnan A, Luscious L (2020) Nanomaterials incorporated electrochemical sensors for the monitoring of pyridoxine: a mini review. Mater Today Proc 46:2998–3004. https://doi.org/10.1016/j.matpr.2020.12.703
Sartori ER, Medeiros RA, Rocha-Filho RC, Fatibello-Filho O (2010) Square-wave voltammetric determination of propranolol and atenolol in pharmaceuticals using a boron-doped diamond electrode. Talanta 81(4–5):1418–1424. https://doi.org/10.1016/j.talanta.2010.02.046
Sharma A, Arya S (2019) Economical and efficient electrochemical sensing of folic acid using a platinum electrode modified with hydrothermally synthesized Pd and Ag Co-Doped SnO2 nanoparticles. J Electrochem Soc 166(13):B1107–B1115. https://doi.org/10.1149/2.0261913jes
Sharma A, Arya S, Chauhan D, Solanki PR, Khajuria S, Khosla A (2020) Synthesis of Au–SnO2 nanoparticles for electrochemical determination of vitamin B12. J Market Res 9(6):14321–14337. https://doi.org/10.1016/j.jmrt.2020.10.024
Shishkanova TV, Sinica A (2022) Electrochemically deposited cobalt bis(dicarbollide) derivative and the detection of neuroblastoma markers on the electrode surface. J Electroanal Chem. https://doi.org/10.1016/j.jelechem.2022.116674
Sonkar PK, Ganesan V, Sen Gupta SK, Yadav DK, Gupta R, Yadav M (2017) Highly dispersed multiwalled carbon nanotubes coupled manganese salen nanostructure for simultaneous electrochemical sensing of vitamin B2 and B6. J Electroanal Chem. https://doi.org/10.1016/j.jelechem.2017.11.050
Sorouraddin MH, Rashidi MR, Ghorbani-Kalhor E, Asadpour-Zeynali K (2005) Simultaneous spectrofluorimetric and spectrophotometric determination of melatonin and pyridoxine in pharmaceutical preparations by multivariate calibration methods. Farmaco 60(5):451–458. https://doi.org/10.1016/j.farmac.2005.03.009
Stach K, Stach W, Augoff K (2021) Vitamin B6 in health and disease. Nutrients. https://doi.org/10.3390/nu13093229
Stanković DM, Kalcher K (2015) The immunosuppressive drug-Rapamycin-electroanalytical sensing using boron- doped diamond electrode. Electrochim Acta 168:76–81. https://doi.org/10.1016/j.electacta.2015.03.200
Tabanlıgil Calam T (2019) Analytical application of the poly(1H–1,2,4-triazole-3-thiol) modified gold electrode for high-sensitive voltammetric determination of catechol in tap and lake water samples. Int J Environ Anal Chem 99(13):1298–1312. https://doi.org/10.1080/03067319.2019.1619716
Vadivaambigai A, Senthilvasan PA, Kothurkar N, Rangarajan M (2015) Graphene-oxide-based electrochemical sensor for salicylic acid. Nanosci Nanotechnol Lett 7(2):140–146. https://doi.org/10.1166/nnl.2015.1909
van Hunsel F, van de Koppel S, van Puijenbroek E, Kant A (2018) Vitamin B6 in health supplements and neuropathy: case series assessment of spontaneously reported cases. Drug Saf 41(9):859–869. https://doi.org/10.1007/s40264-018-0664-0
Vedhi C, Selvanathan G, Arumugam P, Manisankar P (2009) Electrochemical sensors of heavy metals using novel polymer-modified glassy carbon electrodes. Ionics 15(3):377–383. https://doi.org/10.1007/s11581-008-0277-1
Vrolijk MF, Opperhuizen A, Jansen EHJM, Hageman GJ, Bast A, Haenen GRMM (2017) The vitamin B6 paradox: supplementation with high concentrations of pyridoxine leads to decreased vitamin B6 function. Toxicol in Vitro 44:206–212. https://doi.org/10.1016/j.tiv.2017.07.009
Vrolijk MF, Hageman GJ, van de Koppel S, van Hunsel F, Bast A (2020) Inter-individual differences in pharmacokinetics of vitamin B6: a possible explanation of different sensitivity to its neuropathic effects. PharmaNutrition. https://doi.org/10.1016/j.phanu.2020.100188
Waltman RJ, Bargon J (1986) Electrically conducting polymers: a review of the electropolymerization reaction, of the effects of chemical structure on polymer film properties, and of applications towards technology. Can J Chem 64(1):76–95. https://doi.org/10.1139/v86-015
Wright CS, Samitz LMH, Brown H (1943) Vitamin B6 (pyridoxine) in dermatology. Arch Dermatol Syphilol. https://doi.org/10.1001/archderm.1943.01500230041007
Yadav SK, Agrawal B, Goyal RN (2013) AuNPs-poly-DAN modified pyrolytic graphite sensor for the determination of Cefpodoxime Proxetil in biological fluids. Talanta 108:30–37. https://doi.org/10.1016/j.talanta.2013.02.044
Yuqing M, Jianrong C, Xiaohua W (2004) Using electropolymerized non-conducting polymers to develop enzyme amperometric biosensors. Trends Biotechnol 22(5):227–231. https://doi.org/10.1016/j.tibtech.2004.03.004
Zabolestani H, Sarhadi H, Beitollahi H (2021) Electrochemical sensor based on modified screen printed electrode for vitamin b6 detection. Surf Eng Appl Electrochem. https://doi.org/10.3103/S1068375521020149
Zembrzuska D, Kalecki J, Cieplak M, Lisowski W, Borowicz P, Noworyta K, Sharma PS (2019) Electrochemically initiated co-polymerization of monomers of different oxidation potentials for molecular imprinting of electroactive analyte. Sensors Actuators B Chem. https://doi.org/10.1016/j.snb.2019.126884
Zeybek B, Üğe A (2021) Development of carbon-based sensors for electrochemical quantification of vitamins B2 and B6 at nanomolar levels. Chem Pap. https://doi.org/10.1007/s11696-020-01387-9
Zhang P, Tsuchiya K, Kinoshita T, Kushiyama H, Suidasari S, Hatakeyama M, Imura H, Kato N, Suda T (2016) Vitamin B6 Prevents IL-1NL Protein Production by Inhibiting NLRP3 Inflammasome activation. J Biol Chem 291(47):24517–24527. https://doi.org/10.1074/jbc.M116.743815
Zhang, M., Zhao, H. T., Xie, T. J., Yang, X., Dong, A. J., Zhang, H., Wang, J., & Wang, Z. Y. (2017). Molecularly imprinted polymer on graphene surface for selective and sensitive electrochemical sensing imidacloprid. Accessed from. http://www.elsevier.com/open-access/userlicense/1.0/
Zuo X, Zhang H, Li N (2012) An electrochemical biosensor for determination of ascorbic acid by cobalt (II) phthalocyanine-multi-walled carbon nanotubes modified glassy carbon electrode. Sens Actuators B Chem 161(1):1074–1079. https://doi.org/10.1016/j.snb.2011.12.013
Acknowledgements
The authors thank Amrita Vishwa Vidyapeetham for providing the facilities to carry out this research. The characterization helps from the Amrita Center for Nanotechnology, CSIR-Central Electrochemical Research Institute, Karaikudi, CLIF, University of Kerala and Christian College Chengannur are also gratefully acknowledged.
Funding
This research did not receive any specific grant from the public, commercial, or not-for-profit funding agencies.
Author information
Authors and Affiliations
Contributions
SA contributed to Proposed the topic, conducted experiments, organized data, and wrote the original draft. BS contributed to supervised the experiments, scrutinized the data, and edited the whole manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Antherjanam, S., Saraswathyamma, B. Electrochemical preparation and the characterizations of poly(3,5-diamino 1,2,4-triazole) film for the selective determination of pyridoxine in pharmaceutical formulations. Chem. Pap. 77, 4285–4296 (2023). https://doi.org/10.1007/s11696-023-02777-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11696-023-02777-5