Abstract
In this article, we designed one-step economic eco-harmonious microwave-assisted procedure to prepare nitrogen-doped carbon dots. We selected egg white as a cheap glycoprotein-based carbon source without the assistance of any chemicals. The synthetic process requires only 3 min during which carbonization and nitrogen doping are realized at the same time. The fabricated carbon dots were characterized for particle size, structure and photoluminescence behaviour. The nanodots were amorphous carbon-rich naturally nitrogen-doped particles with plentiful attached hydrophilic functional groups. They had average particle size 2.98 ± 1.57 nm, emitted strong blue fluorescence and showed excitation-dependant emission behaviour. What is more, the practical use of this system for raspberry ketone determination in commercially available weight loss dietary supplement product is demonstrated successfully. In ethylene glycol medium, the addition of raspberry ketone enhances the emission intensity of the synthesized carbon dots. The effect of reaction time and solvent was investigated. After optimization, the intensity enhancement was linear to the amount of raspberry ketone added to the assay solution in the concentration range of 100–1000 ng/ml, with detection and quantitation limits of 15.10 and 45.45 ng/ml, respectively. The method was validated in accordance to International Conference on Harmonization (ICH) guidelines and further applied to raspberry ketone capsules showing excellent results.
Graphical abstract

Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Carbon, the king of elements, is found all around us in every living thing. It is the backbone of many materials, being very hard in sparkling diamonds or even very soft graphite in pencil lead. It is very reactive towards different atoms even other carbon atoms, existing on its own as carbon lumps (Sparrow 1999; Saucerman 2005). In the past decades, the carbon nanomaterials series has greatly expanded to include graphite, diamond, graphene, activated carbon, fullerenes, carbon nanotubes and fluorescent nanomaterials (Nasir et al. 2018).
Carbon-based dots (CDs) are zero-dimensional nanoparticles, less than 10 nm in size, showing intrinsic fluorescence (Yang et al. 2013; Himaja et al. 2015; Liu et al. 2019b). CDs were first discovered in 2004 when Xu et al. reported that a mixture of fluorescent nanoparticles appeared during the electrophoretic purification of single-walled carbon nanotubes. These particles showed blue, green, yellow and orange fluorescence under 365 nm UV light. Further characterization showed it was a carbonaceous material that consisted of carboxyl groups (Xu et al. 2004; Himaja et al. 2015).
All CDs consist of sp2/sp3 carbonaceous core with hydrogen- and oxygen-containing functional groups and surrounded sometimes by heteroatoms or other surface modifications (Zhu et al. 2015; Sun and Lei 2017). They possess unique outstanding properties including excellent water dispersion, small size, chemical inertness, low toxicity, intensive multicolour tunable fluorescence, easy eco-friendly preparation and wide (bio)sensing and drug delivery applications. Being composed of intrinsically non-toxic carbon, CDs are biocompatible because they resemble the carbonaceous backbone of biomolecules (Dong et al. 2012; Yang et al. 2013; Himaja et al. 2015; Sun and Lei 2017; Chen et al. 2018).
The preparation of these nanodots includes two synthetic strategies, namely top-down and bottom-up synthesis.
In top-down strategy, CDs are prepared through splitting of bulky carbonaceous materials as graphite, graphene and graphene oxide, carbon nanotubes, nanodiamonds and coal. These starting materials have perfect sp2 clusters with highly delocalized π electrons. That’s why they lack efficient band gaps to show photoluminescence (PL), and size/ surface modification is required to prepare fluorescent CDs (Zhu et al. 2015). This cutting can be done through oxidative cleavage using concentrated acids, hydrothermal, or solvothermal methods for already oxidized carbon sources under high temperature and pressure conditions, electrochemical exfoliation, ultrasound or microwave-assisted methods or high-power laser ablation. These methods are time-consuming, require costly raw materials, special devices and harsh working conditions and use hazardous chemicals. Moreover, the obtained dots are polydisperse and require post-modification steps to boost their PL (Prasannan and Imae 2013; Benítez-Martínez and Valcárcel 2015; Chen et al. 2018; Liu et al. 2019b).
In bottom-up strategy, CDs are prepared through dehydration and then carbonization of small organic precursors or polymers such as glucose, citric acid, ascorbic acid, glycerol and ethylene diamine tetraacetic acid disodium salt (Chen et al. 2018). Any carbon-based molecule can be used as a starting material, either purified commercial chemicals or natural resources. These molecules naturally contain alcohol, carboxyl, carbonyl or amino groups that can dehydrate at high temperature. This can be achieved through conventional hydrothermal methods, plasma treatment, microwave irradiation or ultrasonication (Himaja et al. 2015; Zhu et al. 2015). These synthetic routes are environmentally benign using readily available starting materials along with simple procedures and providing well-controlled shape and size of the product (Benítez-Martínez and Valcárcel 2015; Chen et al. 2018).
The amount of surface-attached functional groups and doped elements greatly affect the luminescence of CDs. Doping of CDs with different heteroatoms such as nitrogen (N), most commonly reported in the literature, significantly enhances their fluorescence behaviour (Sun and Lei 2017).
Although CDs are in fashion in the present-day scenario, there are many challenges ahead. The major issue is developing simple green experimental setups for the synthesis of CDs that maintain their stability and PL, along with eliminating additional surface passivation/doping steps (Himaja et al. 2015). The greenness of the synthetic procedure depends on the correct choice of the starting material and the synthetic conditions.
Unlike conventional heating hydrothermal/solvothermal methods, microwave irradiation causes rapid and uniform bulk heating of the reaction medium. Microwave chemistry relies on the ability of the material to absorb microwave electromagnetic energy and convert it into heat according to its dipole moment, which is known as microwave dielectric heating. Thus, microwave-assisted synthesis shortens the reaction time and improves product quality. In addition, domestic microwave ovens are easily assessable with no need for expensive devices and offer precise control over the reaction parameters without releasing much heat to the environment (Yang et al. 2013; Liu et al. 2014, 2019b; Singh et al. 2019). In light of this, microwave treatment is a promising green synthetic route.
A green starting material should be eco-friendly and cost-effective. To cite a few examples, the literature reports the use of biodegradable waste materials (e.g. waste orange peels (Prasannan and Imae 2013), sugarcane bagasse (Liu et al. 2013), walnut shells (Cheng et al. 2017), waste frying oil (Hu et al. 2014) and eggshell membranes(Wang et al. 2012b)) or commercially available food products (e.g. table sugar (Ansi and Renuka 2018), flour (Qin et al. 2013), milk (Wang and Zhou 2014), coffee beans (Wang et al. 2016) and egg white/yolk (Wang et al. 2012a; Himaja et al. 2015)).
Albumen, commonly known as egg white, is an aqueous protein solution. It contains 88% water and 11% albumin proteins. Ovalbumin is the major component of albumen protein making 54% of the total protein content, followed by ovotransferrin and ovomucin representing 12% and 11%, respectively. These are polypeptides with different carbohydrate chains and disulphide bridges (Abeyrathne et al. 2013; Sunwoo and Gujral 2015). Being carbohydrate- and protein-rich, egg white is an attractive green readily available CD precursor. As per our literature review, reports are published employing egg white in CDs synthesis (Wang et al. 2012a; Zhang et al. 2015; Hu et al. 2016; Baig and Chen 2017). All proposed time/energy-consuming procedures by either prolonged direct heating/hydrothermal or plasma treatment or alkaline hydrolysis of egg white samples.
Herein, our research group proposed a quick, low-cost and eco-friendly one-pot preparation of naturally N-CDs. Our procedure was employed in the kitchen. Egg white was directly treated in a domestic microwave oven. The fabricated CDs were characterized by several analytical techniques to study their particle size, morphology, surface functional groups and optical properties. Further, they were used as fluorescent nanosensors for raspberry ketone (RK) analysis in commercially available dietary supplement product.
Raspberry ketone (RK), 4-(4-hydroxy phenyl) butan-2-one, is a naturally occurring phenolic compound in raspberry fruit. Isolation of low concentrations of this natural RK is very expensive. Hence, it can be produced through chemical or biosynthesis reactions (Bredsdorff et al. 2015). RK has been widely used as a flavouring agent in food products and whitening agent in cosmetics (Lin et al. 2011). Nowadays, it is an important constituent in dietary supplements labelled as slimming agents. Its anti-obesity action is related to its structural similarity to epinephrine. It mediates β-adrenergic stimulation, enhancing lipolysis from white adipocytes and thermogenesis in brown adipocytes (Morimoto et al. 2005). RK held GRAS (Generally Recognized as Safe) status since 1965 for use as a natural flavouring agent. However, this is not the case for synthetic RK which is mainly found in commercially available dietary supplements. The recommended daily intake advised by manufacturers on products’ labels exceed the safe limits (Lee 2016).
To date, few reports have been published for the analysis of RK in dietary supplement products including high-performance liquid chromatographic methods with diode array detection using phenyl columns (Al-othman et al. 2015) and UV detection (Maggi et al. 2016), high-performance thin-layer chromatographic method (Maggi et al. 2016) and IR-spectrophotometric method (Aboul-Enein et al. 2019). Reports on spectrofluorimetric determination of RK in the literature are HPLC fluorescence methods that involved pre-column derivatization of RK with 4-Hydrazino-7-nitro-2, 1, 3-benzoxadiazole (Higashi 2016) or 4-(N,N-dimethylamino sulfonyl)-7-(N-chloro-formylmethyl-N-methylamino)-2,1,3-benzoxadiazole (Higashi 2018) as fluorescence labelling agents. Our research group previously reported a synchronous spectrofluorimetric determination of RK using its native fluorescence (Abdelaal et al. 2021). In this work, the reported fluorescent N-CDs are considered the first nanoprobes for the sensitive detection of RK in dietary supplements. The analysis system measures the fluorescence enhancement of N-CDs at 441 nm caused by RK. Validation followed the ICH guidelines, and the method was further applied to the analysis of RK in commercially available dietary supplement capsules.
Experimental
Materials
Chemicals
Fresh unfertilized chicken eggs bought from the local market; double deionised water employed during the preparation of CDs; HPLC-grade solvents (Sigma-Aldrich, Gmbh, Germany); universal buffer solution prepared as described in literature (Britton and Robinson 1918); analytical grade quinine sulphate, (Sigma-Aldrich, Gmbh, Germany); raspberry ketone working standard (certified purity 99.50%, Baoji Guokang Biotechnology Co. Ltd, China); and the “Raspberry ketones” drug product purchased from an online supplement store, labelled to contain 100 mg RK per capsule.
Standard solutions
Ethanolic RK (100 µg/mL) stock solution was prepared. Further dilution was done using the same solvent to obtain 10 µg/mL standard working solution.
Apparatus
High-resolution transmission electron microscope (HRTEM) images were taken with a JEOL JEM-2100 microscope (Tokyo, Japan) operated at an accelerating voltage 200 kV. To obtain particle size distribution, dynamic light scattering (DLS) measurements were carried out using Malvern ® Zetasizer ver.6.32, Nano-ZS (UK), equipped with a cuvette rotation/ translation unit and a He–Ne laser at scattering angle 90° and 25 °C. The acquisition time for each run was 30 s. The content of CHNS elements was determined using an elemental analyzer vario MACRO cube ®, Elementar, Germany. Fourier transform infrared spectra (FT-IR) were recorded from 4000 to 400 cm−1 on Thermo Scientific ® Nicolet ® iS10 FT-IR spectrometer (Massachusetts, US), using KBr pellets. The UV–Vis absorbance spectra were recorded on Shimadzu® double beam (Kyoto, Japan) 1601 UV–Vis spectrophotometer connected to a computer fitted with UV-PC personal spectroscopy software version (3.7) using matched quartz cuvettes in a thermostatted holder. DESAGA, Sarstedt-Gruppe, MinUVIS UV Lamp (λ = 254 nm /366 nm) was used. The PL spectra of the prepared CDs were recorded using Shimadzu® spectrofluorometer RF-6000 (Kyoto, Japan), with slit width 5 nm for both excitation and emission, and LabSolutions® software (Rev.B.04.01, Shimadzu) was used for data manipulation.
Methods
Procedure for synthesis of N-CDs
Egg white was separated well from fresh egg and treated in a domestic microwave oven (1100 Watts) for 3 min. The brown product was cooled at room temperature and dissolved in deionized water with continuous stirring. The obtained CDs suspension was centrifuged for 20 min at 6000 rpm to discard any unreacted ashes. The resulting brown-yellow solution was dialysed against deionized water for 24 h and then lyophilized to obtain powdered N-CDs.
Quantum yield (QY) measurements
QY was calculated relative to quinine sulphate as a reference standard following the equation:
(Zhuang et al. 2016).where
QY: quantum yield of N-CDs solution in ethylene glycol.
QYS: quantum yield of 0.1 M H2SO4 quinine sulphate solution. ƞ/ƞS: refractive indices of sample and reference solvents; respectively.
Grad/ GradS: slope from the plot correlating integrated fluorescence intensity upon excitation at 360 nm vs. absorbance at the same excitation wavelength of different concentrations of sample and reference, respectively.
Absorbance at the excitation wavelength was kept under 0.1 in order to minimize the re-absorbance effect.
Turn-on detection of RK using the prepared N-CDs
In a set of 10-mL volumetric flasks, different concentrations of RK were prepared in the range of 100–1000 ng/mL in ethylene glycol containing 0.1% N-CDs. Fluorescence intensities were measured at λexcitation/λemission = 360/441 nm. Calibration plot was constructed correlating ΔF between fluorescence intensity of test solutions and blank 0.1% N-CDs solution in ethylene glycol to corresponding RK concentrations.
Determination of RK in dietary supplement capsules
Ten capsules were emptied, and their contents were weighted and ground into fine powder. An amount equivalent to 10 mg RK was weighted, sonicated in 50-mL ethanol for 15 min and then filtered into 100-mL volumetric flask. The volume was completed with ethanol to obtain a 100 µg/mL stock solution. Aliquot volumes were assayed using the previously described procedure, and claimed concentrations were calculated using the corresponding regression equation. Standard addition technique was applied. Known amounts of pure standards were added to commercial samples; those mixtures were then subjected to the entire sample preparation and quantitation steps. Recoveries were calculated by subtracting the concentration of the non-spiked samples from the total concentration of the spiked samples.
Results and discussion
Carbon dots have been a popular platform for sensing applications, thanks to their excellent water dispersion, unique optical properties and low toxicity. Among all synthetic routes reported in the literature, bottom-up synthesis remains famous for its low cost and feasibility for large-scale production. Herein, a one-pot procedure is proposed to prepare N-CDs. We only used a domestic microwave oven and readily available chicken egg white. The reaction time was short without consuming any hazardous solvents. This synthetic procedure is of low cost and low energy/time consumption, unlike other published methods utilizing egg white as precursor (Wang et al. 2012a; Hu et al. 2016; Baig and Chen 2017).
Choice of starting material
According to the literature, N-doping obviously enhances the fluorescence of CDs (Benítez-Martínez and Valcárcel 2015; Sun and Lei 2017). As per our aim to adopt a simple green synthetic procedure along with excellent results, we selected albumen as an environmentally benign readily available material. Its amino acid building blocks can produce highly fluorescent naturally N-doped CDs, eliminating the tedious doping/surface passivation steps.
Mechanism of formation of N-CDs
The mechanism of N-CDs formation may be possibly summarized as follows: The heat produced by microwave irradiation first causes denaturation of the egg white proteins in which uncoiling of the polypeptide chains occurs, and the bonds holding the polypeptides together are disrupted. Then, the resultant amino acids are further dehydrated, polymerized and finally carbonized to yield N-doped CDs having abundant uncarbonized surface functional groups (Zhang et al. 2015; Liu et al. 2019a). Microwave treatment was tested at different time intervals (1, 2, 3, 4 min.). Microwave treatment for 3 min was optimum for allowing all synthetic stages to take place and achieve the smallest-sized particles along with the highest PL. Longer microwave heating produced larger-sized particles that showed poor PL.
Characterization of the prepared N-CDs
HRTEM image (Fig. 1a) shows well-dispersed quasi-spherical particles with diameter 2.98 ± 1.57 nm, obtained by averaging 150 particles. The diffuse ring in selected area electron diffraction revealed that the prepared particles are amorphous (Fig. 1b). According to DLS measurements, particle size of the prepared N-CDs is distributed in the range 1.74–4.85 nm (Fig. 1c). Both results agreed together with some accepted experimental errors. Elemental analysis % results reveal that our prepared dots are composed of C (56.68), H (4.57), N (9.80), S (0.82) and O (calculated, 28.13).
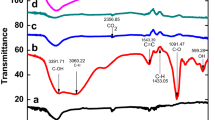
From the FT-IR spectrum (Fig. 2a), we concluded the following information about the grafting of chemical groups in the fabricated N–CDs. A broad band at 3400 cm−1 is due to O–H and N–H stretching vibrations. Symmetrical and asymmetrical –C–H stretching vibrations are seen at 2900 cm−1. Sharp peaks at 1700 and 1650 cm−1 can be assigned to –C = O, –C = C and –C = N vibrations. Peaks at 1560 and 1400 cm−1 correspond to –N–H and –C–H bending vibrations, respectively. Also bands at 1260 and 1080 cm−1 may be due to –C–N and –C–O bonds (Yadav 2005). This indicates that the fabricated N-CDs are carbon-rich with plenty of amino, hydroxyl and carbonyl functionalities attached to the surface.
The UV–Vis absorption spectrum (Fig. 2b) of the prepared N-CDs is typical to that reported in the literature (Zhang et al. 2015). It shows two strong absorption regions. The first absorption band is centred on 204 and 270 nm due to π–π* transitions of –C = C bonds. The appearance of a broad band rather than a sharp peak may be due to size distribution from approximately 2 to 5 nm. The second is a strong absorption tail extended above 300 nm, ascribed to n–π* transitions of –C = O or –C–N bonds. (Zheng et al. 2015; Yuan et al. 2016; Chen et al. 2018; Liu 2020).
N-CDs solution is faint yellow under daylight and emits blue fluorescence under a 365 nm UV lamp, despite that the original albumen sample was non-emissive under UV light. Although the fluorescence origin of CDs has been studied extensively, the exact mechanism remains controversial, and no unified theory is available due to diversity in precursors, synthesis routes and post-treatment procedures. The most popular mechanisms are surface state and core state fluorescence. In surface state fluorescence, a range of attached functional groups can introduce new energy levels, thus new electronic transitions that result in a series of new emissive states. Core state fluorescence occurs due to band gap transitions of electrons within the conjugated π domain. In the carbon core of 3–5 nm particles, energy gap between HOMO and LUMO is too small to contribute in PL of particles. However, carbon core may act as fluorescence quencher due to fluorescence resonance energy transfer. Thus, surface state emission is the most widely accepted mechanism and emission of our synthesized particles are probably due to radiative recombination of the excited electrons from n–π* electronic transitions of surface attached functional groups (Fang et al. 2017; Liu 2020). Excitation spectrum shows two excitation peaks at 263 and 360 nm proving the occurrence of two electronic transitions. When excited at 360 nm, strong emission peak is centred at 441 nm (Fig. 3a). Emission intensity was slightly lower than excitation intensity. This difference is due to vibrational relaxation of the excited N-CDs. The prepared N-CDs showed an excitation-dependant fluorescence behaviour (Fig. 3b). The emission peak was red-shifted with changing fluorescence intensity and peak shape, when the excitation wavelength was increased from 320 to 450 nm. This phenomenon may be due to size distribution or emissive traps on the surface (Zhu et al. 2015; Chen et al. 2018; Hua et al. 2018). In our case, the nanodots showed narrow size distribution and the excitation-dependant PL behaviour can be more probably due to several emissive traps due to several attached functional groups. Even after been stored for 3 months, no significant variation in PL intensity is observed. They were also photostable showing insignificant changes in PL intensity upon continuous excitation at 360 nm. The calculated QY of the prepared N-CDs is 7.93%.
The effect of experimental conditions on N-CDs Fluorescence
N-CDs fluorescence behaviour was investigated in different conditions including dilution of prepared N-CDs stock, solvent used and solution pH.
Dilution effect
Fluorescence intensity of aqueous CDs solutions was measured in six different N-CDs concentrations. Among all measurements, 0.1% N-CDs solution showed the highest fluorescence intensity. Higher concentrations showed nearly diminished fluorescence due to inner filter effects and aggregation caused quenching (Zu et al. 2017) and lower concentrations showed gradual decrease upon dilution (Fig. 4b). Hence, N-CDs were used in the concentration 0.1% throughout the detection experiment. It was also observed that a red-shift in emission wavelength occurred from 441 to 498 nm as the concentration of CDs solution was increased from 0.1 to 0.5%. This observed bathochromic shift can be explained that as the number of particles increases, inter-particle distance decreases, and hydrogen bonding between surface attached hydroxyl and amine groups increases. This electron re-distribution decreases the energy gap between HOMO and LUMO, thus red-shifting of λemission. (Ba et al. 2020; Liu 2020) (Fig. 4a).
Solvent effect
The fluorescence of N-CDs was investigated in different polar solvents including acetone, acetonitrile, water, DMF, ethanol, ethylene glycol and methanol (Fig. 4c). The abundance of hydrophilic surface attached groups makes the fabricated N-CDs freely dispersed in water and other polar organic solvents. The emitted fluorescence of the prepared N-CDs was affected by the solvent used. We found that the highest intensity occurred in the solutions using ethylene glycol and DMF as solvents. As per the solvent greenness, DMF is ranked as reprotoxic hazardous, whereas ethylene glycol is ranked in between recommended and problematic solvents (Byrne et al. 2016). Accordingly, ethylene glycol is regarded as the best solvent where the most enhancement of N-CDs fluorescence is achieved.
pH effect
The influence of pH on fluorescence of the prepared N-CDs was studied using different pH values of Britton–Robinson buffer (Fig. 4d). Fluorescence intensity of the N-CDs prepared by our procedure increases gradually from pH 2 till 8, and then decreases at pH higher than 8. No significant shifting of emission wavelength was observed at different pH values. This agrees with the fact that amine-functionalized CDs are pH sensitive, due to protonation and deprotonation of amino groups. Acidic pH causes the protonation of amino group showing reduced electron-donating activity, whereas basic pH causes electron transfer. This significant change in functional groups prohibits some electronic transitions. Thus, fluorescence is reduced at both basic and acidic conditions (Liu 2020).
Turn-on detection of RK using the prepared N-CDs
Sensing mechanism
Significant enhancement was observed upon scanning UV–Vis absorption (Fig. 5a) and emission spectra of N-CDs (Fig. 5c) in the presence of RK. The possible sensing mechanism may be attributed to the phenomenon of “photo-induced charge transfer”, during which, charge transfer occurs between the fluorophore and the analyte functionalities. A complex formation between receptor and fluorophore is accompanied with changed π conjugation extent. Thus, electronic transitions and accordingly fluorescence signals are altered (Sun and Lei 2017). Our detailed characterization reveals that the fabricated N-CDs have abundant hydroxyl, carboxyl and amino groups on the surface. Their hydrophilicity make them hydrogen bond donors or acceptors with analytes. Regarding the interaction with RK, H-Bonds may possibly be formed between carbonyl moiety of RK and hydroxyls on N-CDs or between phenyl of RK and carboxyl or amino groups on N-CDs (Fig. 5b). These possible interactions give rise to larger chromophores and fluorophores with increased conjugation extent, inhibiting non-radiative recombination and excited electrons return to ground state through radiative recombination only. (Hua et al. 2018; Hu et al. 2022).
(a) UV–Vis spectra of (..-..-..-) Raspberry Ketone, (........) N-CDs in ethylene glycol and (——) N-CDs + Raspberry Ketone (b) Schematic illustration of the possible fluorescence enhancement mechanism (c) emission spectra showing fluorescence enhancement of N-CDs in the presence of raspberry ketone in the concentration range (0–1000 ng/mL)
Optimization of experimental conditions
For determination of the optimal detection conditions, the effect of different solvents and incubation time on the fluorescence increment was studied. The extent of fluorescence enhancement was studied in different solvents including water, ethylene glycol and Robinson–Britton (RB) buffer at pH values of 2.00, 6.00 and 10.00. From the calculated ΔF values, we found that the highest increment was observed in ethylene glycol solutions. ΔF values at different incubation times from 0 to 30 min at 5-min intervals were also investigated. We found that stable fluorescence enhancement was achieved after 20 min. Thus, subsequent RK measurements were performed in ethylene glycol after being incubated for 20 min with N-CDs (Fig. 6).
Method validation
Fluorescence of N-CDs was significantly enhanced as the concentration of RK increases, with negligible effect on peak shape. Validation was done in accordance to ICH guidelines. (Validation of Analytical Procedures: Text and Methodology Q2(R1) 2005). ΔF (λ excitation/ λ emission 360/441 nm) is linearly related to RK concentration (C) in the range 100.00–1000.00 ng/mL. The computed regression equation was ΔF = 8.7057 C + 251.9124, with a correlation coefficient (r) = 0.9993. LOD and LOQ values are 15.10 and 45.45 ng/mL, respectively. Further, the method was applied for the analysis of pure RK samples showing good accuracy and precision. Assay validation parameters are shown in Table 1. The results are compared favourably with those from published HPLC method (Maggi et al. 2016), using student’s t test and variance ratio F-test. The comparison showed no statistical difference, as shown in Table 2.
Application of the proposed method to the analysis of RK in dietary supplement capsules
To evaluate the analytical performance of the developed method, the method was used for the assay of RK in dietary supplement capsules. Excellent average per cent recoveries were obtained. Standard addition technique was also applied to assess the validity of the extraction method. Results are shown in Table 3.
Conclusions
In summary, an easy commercially feasible green bottom-up method has been adopted to prepare naturally N-doped CDs through direct microwave irradiation of albumen. The as-synthesized CDs showed narrow size distribution in the range 1.74–4.85 nm and were found to emit blue fluorescence with QY 7.93% at excitation wavelength 360 nm. Their self-passivated layer facilitates water dispersion and efficient PL. The system was proved successful for the sensitive assay of RK through detection of fluorescence turn-on signals. When RK commercially available capsules were analysed, the results were in excellent agreement with the labelled amounts. Thus, we hope that our work would leave a mark in the development and application of these rising star nanoparticles.
References
Abdelaal SH, El NF, Hassan SA, El-kosasy AM (2021) Quality control of dietary supplements : an economic green spectrofluorimetric assay of Raspberry ketone and its application to weight variation testing. Spectrochim Acta Part A Mol Biomol Spectrosc 261:120032–120038. https://doi.org/10.1016/j.saa.2021.120032
Abeyrathne EDNS, Lee HY, Ahn DU (2013) Egg white proteins and their potential use in food processing or as nutraceutical and pharmaceutical agents: a review. Poult Sci 92:3292–3299
Aboul-Enein HY, Antochi OM, Nechifor G, Bunaciu AA (2019) Analysis of raspberry ketone in nutraceutical formulation using fourier transform infrared spectrophotometric method. Open Bioact Compd J 7:8–13. https://doi.org/10.2174/1874847301907010008
Al-othman ZA, Al-warthan A, Aboul-enein HY et al (2015) Mechanistic approaches of PhE and PPF columns separation for rasberry ketone and caffeine. J Liq Chromatogr Relat Technol 38:1324–1332. https://doi.org/10.1080/10826076.2015.1048874
Ansi VA, Renuka NK (2018) Table sugar derived carbon dot – a naked eye sensor for toxic Pb 2 + ions. Sens Actuators B Chem 264:67–75. https://doi.org/10.1016/j.snb.2018.02.167
Ba XX, Zhang L, Yin YL et al (2020) Luminescent carbon dots with concentration-dependent emission in solution and yellow emission in solid state. J Colloid Interface Sci 565:77–85. https://doi.org/10.1016/j.jcis.2020.01.007
Baig MMF, Chen YC (2017) Bright carbon dots as fluorescence sensing agents for bacteria and curcumin. J Colloid Interface Sci 501:341–349. https://doi.org/10.1016/j.jcis.2017.04.045
Benítez-Martínez S, Valcárcel M (2015) Graphene quantum dots in analytical science. TrAC - Trends Anal Chem 72:93–113. https://doi.org/10.1016/j.trac.2015.03.020
Bredsdorff L, Wedebye EB, Nikolov NG et al (2015) Raspberry ketone in food supplements - High intake, few toxicity data: A cause for safety concern ? Regul Toxicol Pharmacol 73:196–200. https://doi.org/10.1016/j.yrtph.2015.06.022
Britton HTS, Robinson RA (1918) Universal buffer solutions and the dissociation constant of veronal. J Chem Soc 1456–1462
Byrne FP, Jin S, Paggiola G et al (2016) Tools and techniques for solvent selection: green solvent selection guides. Sustain Chem Process 4:7–30. https://doi.org/10.1186/s40508-016-0051-z
Chen W, Lv G, Hu W et al (2018) Synthesis and applications of graphene quantum dots: a review. Nanotechnol Rev 7:157–185
Cheng C, Shi Y, Li M et al (2017) Carbon quantum dots from carbonized walnut shells: Structural evolution, fl uorescence characteristics, and intracellular bioimaging. Mater Sci Eng C 79:473–480. https://doi.org/10.1016/j.msec.2017.05.094
Dong Y, Shao J, Chen C et al (2012) Blue luminescent graphene quantum dots and graphene oxide prepared by tuning the carbonization degree of citric acid. Carbon N Y 50:4738–4743. https://doi.org/10.1016/j.carbon.2012.06.002
Fang Q, Dong Y, Chen Y et al (2017) Luminescence origin of carbon based dots obtained from citric acid and amino group-containing molecules. Carbon N Y 118:319–326. https://doi.org/10.1016/j.carbon.2017.03.061
Higashi Y (2016) Simple HPLC-fluorescence determination of raspberry ketone in fragrance mist after pre-column derivatization with 4-Hydrazino - 7- nitro - 2, 1, 3 - benzoxadiazole. J Anal Sci Methods Instrum 6:44–49
Higashi Y (2018) Improved method for determination of raspberry ketone in fragrance mist by HPLC-fluorescence analysis after pre-column derivatization with ( N -chloroformylmethyl- N -methylamino ). J Anal Sci Methods Instrum 8:17–24. https://doi.org/10.4236/jasmi.2018.82002
Himaja AL, Karthik PS, Singh SP (2015) Carbon dots : the newest member of the carbon nanomaterials family. Chem Rec 15:595–615. https://doi.org/10.1002/tcr.201402090
Hu Y, Yang J, Tian J et al (2014) Waste frying oil as a precursor for one-step synthesis of sulfur-doped carbon dots with pH-sensitive photoluminescence. Carbon N Y 77:775–782. https://doi.org/10.1016/j.carbon.2014.05.081
Hu X, An X, Li L (2016) Easy synthesis of highly fl uorescent carbon dots from albumin and their photoluminescent mechanism and biological imaging applications. Mater Sci Eng C 58:730–736. https://doi.org/10.1016/j.msec.2015.09.066
Hu J, Sun Y, Aryee AA et al (2022) Mechanisms for carbon dots-based chemosensing, biosensing, and bioimaging: a review. Anal Chim Acta 1209:338885–338898. https://doi.org/10.1016/j.aca.2021.338885
Hua J, Jiao Y, Wang M, Yang Y (2018) Determination of norfloxacin or ciprofloxacin by carbon dots fluorescence enhancement using magnetic nanoparticles as adsorbent. Microchim Acta 185:1–9. https://doi.org/10.1007/s00604-018-2685-x
Lee J (2016) Further research on the biological activities and the safety of raspberry ketone is needed. NFS 2:15–18. https://doi.org/10.1016/j.nfs.2015.12.001
Lin VC, Ding H, Kuo S et al (2011) Evaluation of in vitro and in vivo depigmenting activity of raspberry ketone from rheum officinale. Int J Mol Sci 12:4819–4835. https://doi.org/10.3390/ijms12084819
Liu M (2020) Optical Properties of Carbon Dots : A Review. Nanoarchitectonics 1:1–12
Liu X, Guo M, Huang J, Yin X (2013) Improved fluorescence of carbon dots prepared from bagasse under alkaline hydrothermal conditions. BioResources 8:2537–2546
Liu Y, Xiao N, Gong N et al (2014) One-step microwave-assisted polyol synthesis of green luminescent carbon dots as optical nanoprobes. Carbon N Y 68:258–264. https://doi.org/10.1016/j.carbon.2013.10.086
Liu ML, Chen B, Li M (2019a) Carbon dots: synthesis, formation mechanism, fluorescence origin and sensing applications. Green Chem 21:449–471. https://doi.org/10.1039/c8gc02736f
Liu ML, Bin CB, Li CM, Huang CZ (2019b) Carbon dots: synthesis, formation mechanism, fluorescence origin and sensing applications. Green Chem 21:449–471. https://doi.org/10.1039/c8gc02736f
Maggi F, Ricciutelli M, Iannarelli R et al (2016) Analysis of Food Supplement with unusual raspberry Ketone Content. J Food Process Preserv 41:13–19. https://doi.org/10.1111/jfpp.13019
Morimoto C, Satoh Y, Hara M et al (2005) Anti-obese action of raspberry ketone. Life Sci 77:194–204. https://doi.org/10.1016/j.lfs.2004.12.029
Nasir S, Hussein MZ, Zainal Z, Yusof NA (2018) Carbon-based nanomaterials/allotropes : a glimpse of their synthesis, properties and some applications. Materials (basel) 11:295–318. https://doi.org/10.3390/ma11020295
Prasannan A, Imae T (2013) One-pot synthesis of fluorescent carbon dots from orange waste peels. Ind Eng Chem Res 52:15673–15678. https://doi.org/10.1021/ie402421s
Qin X, Lu W, Asiri AM et al (2013) Microwave-assisted rapid green synthesis of photoluminescent carbon nanodots from flour and their applications for sensitive and selective detection of mercury ( II ) ions. Sens Actuators B Chem 184:156–162
Saucerman L (2005) Understanding the elements of the periodic table: Carbon., First edit. The Rosen publishing group Inc.
Singh RK, Kumar R, Singh DP et al (2019) Progress in microwave-assisted synthesis of quantum dots ( graphene / carbon / semiconducting ) for bioapplications : a review. Mater Today Chem 12:282–314. https://doi.org/10.1016/j.mtchem.2019.03.001
Sparrow G (1999) The elements: carbon. Benchmark books, Marshall Cavendish
Sun X, Lei Y (2017) Fluorescent carbon dots and their sensing applications. Trends Anal Chem 89:163–180. https://doi.org/10.1016/j.trac.2017.02.001
Sunwoo HH, Gujral N (2015) Chemical Composition of Eggs and Egg Products. Handbook of Food Chemistry. Springer- Verlag, Berlin Heidelberg, pp 331–363
Validation of Analytical Procedures: Text and Methodology Q2(R1) U food and drug administration (2005) International Conference on Harmonisation, Validation of Analytical Procedures: Text and Methodology Q2(R1), US food and drug administration
Wang L, Zhou HS (2014) Green synthesis of luminescent nitrogen-doped carbon dots from milk and its imaging application. Anal Chem 86:8902–8905
Wang J, Wang C, Chen S (2012a) Amphiphilic egg-derived carbon dots: rapid plasma fabrication, pyrolysis process, and multicolor printing patterns. Angew Chemie Int Ed 51:9297–9301. https://doi.org/10.1002/ange.201204381
Wang Q, Liu X, Zhang L, Lv Y (2012b) Microwave-assisted synthesis of carbon nanodots through an eggshell membrane and their fluorescent application. Analyst 137:5392–5397. https://doi.org/10.1039/c2an36059d
Wang L, Li W, Wu B et al (2016) Facile synthesis of fluorescent graphene quantum dots from coffee grounds for bioimaging and sensing. Chem Eng J 300:75–82. https://doi.org/10.1016/j.cej.2016.04.123
Xu X, Ray R, Gu Y et al (2004) Electrophoretic analysis and purification of fluorescent single-walled carbon nanotube fragments. J Am Chem Soc 126:12736–12737
Yadav LDS (2005) Organic Spectroscopy. springer science + Business Media Dordrecht
Yang Z, Li Z, Xu M et al (2013) controllable synthesis of fluorescent carbon dots and their detecetion application as nanoprobes. Nano-Micro Lett 5:247–259
Yuan Y, Zhao X, Qiao M et al (2016) Determination of sunset yellow in soft drinks based on fluorescence quenching of carbon dots. Spectrochim Acta Part A Mol Biomol Spectrosc 167:106–110. https://doi.org/10.1016/j.saa.2016.05.038
Zhang Z, Sun W, Wu P (2015) Highly photoluminescent carbon dots derived from egg white: facile and green synthesis, photoluminescence properties, and multiple applications. ACS Sustain Chem Eng 3:1412–1418. https://doi.org/10.1021/acssuschemeng.5b00156
Zheng XT, Ananthanarayanan A, Luo KQ, Chen P (2015) Glowing graphene quantum dots and carbon dots: properties syntheses and biological applications. Small 11:1620–1636. https://doi.org/10.1002/smll.201402648
Zhu S, Song Y, Zhao X et al (2015) The photoluminescence mechanism in carbon dots (graphene quantum dots, carbon nanodots, and polymer dots ): current state and future perspective. Nano Res 8:355–381. https://doi.org/10.1007/s12274-014-0644-3
Zhuang Q, Wang Y, Ni Y (2016) Solid-phase synthesis of graphene quantum dots from the food additive citric acid under microwave irradiation and their use in live-cell imaging. Luminescence 31:746–753. https://doi.org/10.1002/bio.3019
Zu F, Yan F, Bai Z et al (2017) The quenching of the fluorescence of carbon dots: a review on mechanisms and applications. Microchim Acta 184:1899–1914. https://doi.org/10.1007/s00604-017-2318-9
Funding
Open access funding provided by The Science, Technology & Innovation Funding Authority (STDF) in cooperation with The Egyptian Knowledge Bank (EKB). No funding was received for conducting this study.
Author information
Authors and Affiliations
Contributions
All authors contributed to study conception and design. Data collection and methodology were performed by Sarah H. Abdelaal. Original draft was written by Sarah H. Abdelaal. Writing review and editing were done by Amira M. El-Kosasy and Mona H. Abdelrahman, and supervision was done by Amira M. El-Kosasy.
Corresponding author
Ethics declarations
Conflict of interest
The authors have no conflict of interests to declare that are relevant to the content of this article.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Abdelaal, S.H., El-Kosasy, A.M. & Abdelrahman, M.H. One-pot synthesis of N-doped carbon dots from microwave-irradiated egg white: application to raspberry ketone assay by photo-induced charge transfer fluorescence sensing. Chem. Pap. 77, 3867–3879 (2023). https://doi.org/10.1007/s11696-023-02748-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11696-023-02748-w