Abstract
In Alzheimer's disease (AD), neuroinflammation is detrimental in causing neurodegeneration. In the central nervous system, inhibitor of nuclear factor kappa B kinase subunit beta (IKK2/IKKβ/IKKB/IKBKB) signaling is linked to neuroinflammation-mediated learning and memory deficits through canonical pathway, while dopamine agonists have been known to reverse such effects. Our in silico analysis predicted if dopaminergic agonists could have IKKB inhibitory actions, to ameliorate neuroinflammation-associated learning and memory deficits. Here, the FDA-approved Zinc 15 database was screened with IKKB (PDB ID 4KIK). Potential molecules with IKKB inhibition were identified through docking, which also possessed dopaminergic activity. Molecular mechanics—generalized Born and surface area (MMGBSA), induced fit docking (IFD) and molecular dynamic (MD) studies of 100 ns simulation time were done. Apomorphine and rotigotine showed greater non-bonding and bonding interactions with amino acids of IKKB as compared to Aripiprazole in docking studies. The IFD studies predicted improved interactions with IKKB. MMGBSA scores indicated that the complex binding free energies were favorable, and MD studies showed an acceptable root mean square deviation between protein and ligands. The protein–ligand interactions showed hydrogen bonds, water and salt bridges necessary for IKKB inhibition, as well as solvent system stability. On the protein–ligand contact map, the varying color band intensities represented the ligand’s ability to bind with amino acids. Dopamine agonists apomorphine, rotigotine, and aripiprazole were predicted to bind and inhibit IKKB in in silico system.
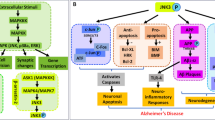
Graphical Abstract

Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Alzheimer's disease (AD), a disorder that involves neurodegeneration, is known for difficulties with cognitive abilities in the elderly. Not only the neurofibrillary tangles (NFTs) but also the senile plaques (SPs) constitute the main characteristics of AD. While SPs contain the 4kD amyloid beta (Aβ) protein, the NFTs are comprised of hyperphosphorylated tau protein (p-tau). Therefore, Aβ aggregation could be the first sign of AD pathogenesis. Aβ cleaved at 42 site (Aβ1–42) serves as a crucial component in the aggregation that contributes to the deposits in SPs (amyloid cascade theory) (Golde et al. 2006). In addition to the Aβ oligomers and NFTs, the participation of glial cells like microglia and astrocytes mediate the release of inflammatory cytokines causing neuroinflammation (Satarker et al. 2022a). Thus, there is a significant need for anti-AD therapies as early clinical investigations of medication targeting Aβ pathways have failed. As a result, reducing neuroinflammation may be an important strategy in such conditions.
A crucial transcriptional controller of the inflammatory response is NF-kB. NF-kB signaling in microglia, astrocytes, and neurons causes a loop of inflammatory events that results in neuronal death in AD (Ju Hwang et al. 2017). The inhibitor of nuclear factor kappa-β (Ikβ) kinase (IKK) complex regulates the NF-kB secretion. Three subunits make up the IKK complex: IKKα, IKKB, and IKKγ, two catalytic subunits, and IKKγ, a regulatory subunit. IKKB has 20–50-fold greater kinase activity for IKBα than IKKα. When bound with its inhibitor, IkBα, NF-kB remains in the cytosol at resting phases. IKK complex activity is necessary for NF-kB activation and relies on the catalytic subunit of the IKKB. Neuromodulators like cytokines and lipopolysaccharide (LPS) trigger IKKB, is instrumental in the dissociation of NF-kB from IkBα. This released NF-kB enters the nucleus, thus causing transcription of various neuroinflammatory genes and producing cytokines like IFN-γ, TNF-α, IL-8, IL-6, and IL-2, along with nitric oxide synthase (iNOS). Thus, IKKB activates the canonical pathway of NF-kB upon Aβ exposure (Mudgal et al. 2020; Veerappan et al. 2017; Zhang et al. 2010) Innate immune cells, particularly monocytes and macrophages, interact with LPS to cause an inflammatory response (Chaiwut and Kasinrerk 2022). LPS is a substance found in gram-negative bacteria's outer membrane. It is known to primarily function through TLR signaling (Basu Mallik et al. 2022) via myeloid differentiation factor 88 (MyD88). Stresses like transient ischemia, high mobility group box 1 (HMGB1) toxic substances Pathogen-associated molecular patterns (PAMPs), damage- or danger-associated molecular patterns (DAMPs), aging, reactive oxygen species, and α-synuclein may induce the inflammatory signaling (Frank et al. 2015).
MyD88 activates the IL-1 receptor-associated kinase (IRAKs), which triggers the phosphorylation of tumor necrosis factor receptor (TNFR)-associated factor 6 (TRAF6). This leads to the activation of transforming growth factor-β activated kinase (TAK1) and potentiation of the IKK complex followed by NF-kB translocation and transcription of NLRP3, caspases, and proinflammatory cytokines (Kinra et al. 2021). The NLRP3 is involved in NLRP3 inflammasome formation and facilitates the inflammatory mechanism for the production of beta-site APP cleaving enzyme (BACE1), GSK3β, TNFR1 associated death domain protein (TRADD), NFT, Aβ and development of Tau hyperphosphorylation progressing to AD.
Small pleiotropic signaling proteins known as cytokines are often released by the immune system, cells such as monocytes, macrophages, lymphocytes, and vascular endothelial cells in response to infections or damage. Some of the cytokines include tumor necrosis factors, interleukins, chemokines, interferons, cell stimulating factors, and neurotrophins. They are naturally produced by stimulated neuronal and glial cells in the brain and are implicated in AD (Krueger 2008). DA and their agonist inhibit various mediators of inflammatory mechanisms including MyD88, TRAF6, TAK1, NF-kB, and NLRP3 inflammasome (Gurram et al. 2022).
Dopaminergic receptors namely dopamine receptors (DR) 1, DR2, DR3, DR4, and DR5 are implicated in the modulation of neuroinflammation in the CNS. Recently we reviewed the consequences of DA stimulation for the strategic treatment of neuroinflammation associated with AD (Gurram et al. 2022). Previous reports have shown that D1R agonist SKF81297 and D2R agonist UNC9994 attenuate neuroinflammation and enhance cognitive performance (Liu et al. 2018b; Park et al. 2016). The mammalian target of rapamycin (mTOR), which interferes with the processing of cognition, may be activated by D3 receptors (Millan et al. 2020). D4 agonist RO-10-5824 enhanced cognitive performance in object recognition and Y-maze test in LPS administered rats (Navakkode et al. 2017). Interestingly, the dopaminergic system has also been associated with IKKB signaling in the CNS. An IKKB inhibitor termed Compound A at 1 mg/kg for a week substantially reduces the loss of DA neurons caused by LPS-induced neurotoxicity (Zhang et al. 2010). Literature reported that dopamine and their agonists inhibit various role players at different levels of canonical NF-kB and NLRP3 inflammasome pathway including IRAKs, TRAF6, TAK1, NF-kB, NLRP3 inflammasome, and AD proteins including BACE1, GSK3β (Li et al. 2022; Wu et al. 2020).
Therefore, through this study we attempted to understand via in silico evaluation, the molecular docking attributes of IKKB with Zinc 15 FDA-approved compounds to identify molecules with potential IKKB inhibitory activity, which are previously known to activate the dopaminergic system, in the efforts of developing better treatment strategies towards managing AD.
Materials and methods
Preparation of protein
The protein structure of IKKB was identified and downloaded from the protein data bank (PDB ID: 4KIK). The protein structure was processed with a ‘protein preparation’ setup. The missing side chains and loops were filled, bond orders were assigned, zero bond orders were created to metals, and disulfide bonds were established. The protein was optimized with PROPKA pH of 7.4 ± 2 and water molecules were eliminated. The heavy atoms were converged to 0.30 Å and the protein was subjected to restraint minimization with OPLS3e force field (Satarker et al. 2022b).
Preparation of ligands
A library of Zinc 15 FDA-approved compounds was selected as ligands. They were processed in ‘LigPrep’ available in Maestro Schrodinger. ‘Epik’ was used for generating different possible states at pH of 7.4 ± 2. The ligands were desalted, and a tautomer generation was performed. The stereoisomeric computation involved the generation of a maximum of 32 chiral structures per ligand, from the 3D structures of the ligands. (Madhavi Sastry et al. 2013).
Rigid molecular docking
The GLIDE ligand docking function in Maestro Schrodinger was used for docking. (Halgren et al. 2004) We eliminated the ligands containing more than 500 atoms and 100 rotatable bonds. The van der Waals radii with a scaling factor of 0.80 were employed along with a partial charge cutoff of 0.15. The Zinc 15 FDA-approved library upon preparation was docked in High Throughput Virtual Screening (HTVS), Standard Precision (SP), and Extra Precision (XP) modes. The docking scores included the Epik state penalties. The results were screened and exclusively the dopamine agonists were evaluated. The shortlisted dopamine agonist molecules from XP docking were evaluated for their XP G score, bonding interactions, and non-bonding interactions.
Induced fit docking (IFD)
We used the IFD module in Maestro Schrodinger for this study. A standard docking protocol was employed that generated up to 20 poses. The box was created in the centroid of the ligand. The ring conformations were sampled with an energy window of nearly 2.5 Kcal/mol. The side chains were optimized. The top dopamine agonist molecules were subjected to IFD studies (Sankhe et al. 2021). The bonding, non-bonding interactions, and IFD scores were analyzed for their capacity to show interaction with the orthosteric binding pocket of IKKB.
MMGBSA
The mean molecular generalized Born surface area (MMGBSA) was done to ascertain the binding free energies of the complexes. We used VSGB solvent model to perform MMGBSA (Li et al. 2011). The molecules were screened using the PRIME module of Maestro Schrodinger which evaluates the MMGBSA of the complexes.
Molecular dynamics (MD)
The MD studies provide information on the stability of complexes in the solvent system. Using the Desmond system builder, we generated a TIP3P solvent model. In the MD simulation studies, we used the latest OPLS3e force field developed by Schrodinger Inc., to model the proteins and ions. (Baby et al. 2021). An orthorhombic box was created of size 10 Å x 10 Å x 10 Å using the buffer size method to calculate the size of the box and the box volume was minimized. NaCl salt was added at a concentration of 0.15M where Na+ was the positive ion and Cl− was the negative ion. The Desmond Molecular Dynamics module was used to run this system for the run time of 100ns calculating about 1000 frames. The temperature of 300 K and a pressure of 1 bar were established during the molecular dynamic simulation with NPT ensemble class. Finally, the model system was relaxed, and the simulation was started. After MD studies, we subjected the molecules to MMGBSA analysis and calculated the binding free energies for every 10th frame for a total of 1000 frames of the simulation (Ray et al. 2022)
Results
Molecular docking
The Zinc 15 FDA-approved library upon docking with IKKB predicted 1298 molecules in HTVS, 750 molecules in SP, and 500 molecules in XP mode. Lapatinib showed the highest XP Gscore of −13.015 Kcal/mol. Our prime interest was to identify top inhibitors for IKKB having dopamine receptor agonistic activity (Supplementary Table 1). Therefore, we selected 3 such top molecules obtained in XP docking namely apomorphine, rotigotine, and aripiprazole with XP Gscores of −9.967, −9.039, and −8.067 Kcal/mol, respectively (Fig. 1).
Apomorphine showed 2 bonding interactions through hydrogen bonds with Glutamic acid (Glu) 97 and Cysteine (Cys) 99 and 11 non-bonding interactions with Aspartate (Asp) 103, Asp 166, Tyrosine (Tyr) 98, Glycine (Gly) 102, Methionine (Met) 96, Leucine (Leu)21, Valine (Val) 29, Val 152, Threonine (Thr) 23 and Isoleucine (Ile) 165, and Alanine (Ala) 42 as shown in Fig. 2.
Rotigotine predicted 2 hydrogen bond mediated bonding interactions with Cys 99 and Glu 97, a salt bridge with Asp 103, and 11 non-bonding interactions with Asp 103, Asp 166, Tyr 98, Gly 102, Met 96, Leu 21, Val 29, Val 152, Thr 23, and Ile 165 and Ala 42 as shown in Fig. 3.
Aripiprazole interacted with a single bonding interaction using Cys 99 via hydrogen bonding and 14 non-bonding interactions with Glu 97, Asp 103, Asp 166, Tyr 98, Gly 102, Met 96, Leu167, Leu21, Val 29, Val 152, Thr 23, Ile 165, Ala 42, Tyr 108 as shown in Fig. 4.
Induced fit docking
Apomorphine predicted 16 different poses with IKKB where pose 1 had the highest IFD score of −1466.82 Kcal/mol as shown in Supplementary Table 2. Their analysis revealed that pose 9 with an IFD score of −1464.13 Kcal/mol showed the maximum amount of desirable bonding interactions and non-bonding interactions with IKKB. It predicted a total of 3 hydrogen bonding interactions namely two with Cys 99, and one with Glu 97. ASP 103 showed a single salt bridge with IKKB. It had 9 non-bonding interactions with Tyr 98, Gly 102, Met 96, Leu 21, Val 29, Val 152, Thr 23, Ile 165, and Ala 42 as shown in Fig. 5.
Rotigotine predicted 10 poses in IFD with IKKB as shown in Supplementary Table 3. Pose 1 attained the highest IFD score of −1466.82 Kcal/mol. Our analysis showed that pose 5, with an IFD score of − 1465.21 Kcal/mol was the desired one due to its ability to predict 3 hydrogen bonding interactions with Glu 97, Cys 99, and ASP 103, and 1 salt bridge interaction with ASP 103. It also possessed 9 non-bonding interactions with Tyr 98, Gly 102, Met 96, Leu 21, Val 29, Val 152, Thr 23, Ile 165, and Ala 42 as shown in Fig. 6.
Aripiprazole predicted 12 poses in IFD with IKKB as shown in Supplementary Table 4. Pose 1 attained the highest IFD score of − 1467.39 Kcal/mol. Upon analysis, we found that pose 2, with an IFD score of − 1466.76 Kcal/mol was the desired one due to its ability to predict 2 hydrogen bonding interactions with Asp166, ASP 103, and one salt bridge interaction with ASP 166. Halogen bonds in terms of chloride interactions were observed with Glu175 and Gly25. It also possessed 5 IKKB inhibitory non-bonding interactions with Cys 99, Tyr 98, Leu 21, Thr 23, and Ile 165, as shown in Fig. 7.
MMGBSA
The MMGBSA scores of apomorphine, rotigotine and aripiprazole in complex with IKKB have been depicted in Table 1.
Molecular dynamics
RMSD plots
It was interesting to see that a favorable contact was observed with apomorphine and IKKB throughout 100ns simulation time. The ligand RMSD was seen to be fluctuating from 1.2 to 4.0 Å. For the initial 25 seconds (s), the ligand RMSD averaged at about 1.2 Å, while for the simulation time of 25s to nearly 42 s, the RMSD rose to about 3Å. The protein RMSD ranged from 2.4 to 4.8 Å and was stabilized at an average RMSD of nearly 3 Å as shown in Fig. 8. This could be suggestive that the IKKB–apomorphine complex could be stable in biological conditions.
The interaction of IKKB and rotigotine predicted ligand RMSD ranging from 1.2 to 4.8 Å . During the initial 20ns of the simulation, larger fluctuations were noted with the average RMSD approximately nearing 3.2 Å, while later it was seen to average at about 2.8Å. Similarly, protein RMSD values ranged from 2.4 Å to 5.6 Å, thus having an average RMSD of about 4 Å as shown in Fig. 9.
The interaction of IKKB and Aripiprazole predicted ligand RMSD ranging from 1.9 to 5.3Å. During the initial 30ns of the simulation, better contact was observed with IKKB and aripiprazole evident with lesser RMSD difference between the two as shown in Fig 10. The average ligand RMSD was 2.5 Å. The RMSD of IKKB ranged from 2.7 to 5.5 Å averaged at nearly 3.8 Å. Even though the complex attained the best stability before 30ns, it was seen to sustain acceptable stability during the remaining 70 ns simulation time.
Protein–ligand contacts histogram
The interaction of IKKB–apomorphine predicted hydrogen bonds, hydrophobic bonds, water bridges, and ionic bonds. Primarily, hydrogen bonds and water bridges were observed with Thr 23 and Cys 99 while Glu 97 showed strong hydrogen bonding. Asp 166 prominently showed an ionic bridge along with a water bridge as shown in Fig. 11.
The criteria for scoring a protein–ligand H-bond are as follows:
-
(i)
The distance between the donor and acceptor atoms should be 2.5 Å,
-
(ii)
Donor angle between the donor–hydrogen-acceptor atoms should be of ≥ 120°.
-
(iii)
Acceptor angle between the hydrogen-acceptor-bonded atom should be of ≥ 90°.
The criteria for scoring a hydrophobic interaction are as follows:
-
(i)
π–π: Two aromatic groups stacked face-to-face or face-to-edge
-
(ii)
π cation: Aromatic and charged groups should be within 4.5 Å.
-
(iii)
Other: Any other unspecified hydrophobic sidechain lying within 3.6 Å of an aliphatic or aromatic carbon of a ligand.
The criteria for scoring ionic or polar interactions are as follows:
-
(i)
Two atoms having opposing charges lie within 3.7 Å of one another and no hydrogen bond is formed.
-
(ii)
Ionic interactions can be mediated by side chains or a protein backbone.
The criteria for scoring a protein–water or water–ligand H-bond are as follows:
-
(i)
The distance between the donor and acceptor atoms must be nearly 2.8 Å.
-
(ii)
The donor angle that lies between the donor–hydrogen-acceptor atoms must be ≥ 110°.
-
(iii)
The acceptor angle between the hydrogen-acceptor-bonded atoms must be ≥ 90°.
The IKKB–rotigotine complex majorly showed hydrogen bonding with Glu 97 and Cys 99. Interestingly, Asp 103 showed a higher interaction fraction due to multiple types of bonding namely hydrogen bonds, ionic bonds, and water bridges. The Ile 165 showed hydrophobic bonding as seen in Fig. 12.
The IKKB–Aripiprazole complex majorly showed hydrogen bonding with Glu149, Lys106 Leu21 andAsp103. Hydrophobic bonds are shown majorly with Lys 147, Tyr98, and Leu21. One ionic bond with Asp166 and water bridges with Asp103, Leu21, and Lys106 as seen in Fig. 13.
Protein–ligand contacts timeline
The counts of contacts in correlation to the amino acids are shown in the form of a protein ligand (PL) contact timeline. In the IKKB–apomorphine complex, Cys showed a higher number of contacts as evident by the darker bands as seen in Fig. 14 but were seen to repeatedly gain and lose these contacts. In contrast to the Cys 99, the Glu 97 showed highly consistent contacts throughout 100ns. Similarly, Thr 23 showed consistent contacts post-18ns of the simulation time. It was also noted that Asp 166 showed significant contacts.
The IKKB–rotigotine complex predicted significant contacts with Glu 97 until nearly 90 ns. The Cys 99 predicted more than one contact majorly beyond 90ns simulation time as shown in Fig. 15. Asp 103 also showed significant contacts in the 100ns simulation time.
The IKKB–Aripiprazole complex predicted significant interactions with ASP 166 as compared to other amino acids. Glu 149 and Lys 147 also showed prominent interactions evident through the darker band intensities. Even though interactions with Asp 103, Lys 106, Leu 21, and Tyr 98 were seen to be formed, they were not much significant which is evident with lighter band intensities as shown in Fig. 16.
Post-MD MMGBSA studies
The MMGBSA of the complexes performed after MD studies predicted stable binding free energies. It (MMGBSA dG bind) was calculated at every 10th frame for a total of 1000 frames. The dG bind of apomorphine ranged from − 67.1544 to − 46.226 Kcal/mol, while rotigotine showed lower energies between − 64.1544 and − 46.2954 Kcal/mol. Aripiprazole MMGBSA lied between − 74.0915 and − 48.8436 Kcal/mol for the duration of the simulation. The average MMGBSA DG bind scores are mentioned in Table 2.
Apomorphine
See Fig. 17.
Rotigotine
See Fig. 18.
Aripiprazole
See Fig. 19.
Discussion
The human body contains a dimeric IKKB that has 1–664 amino acids with chains A and B. Chain B possesses phosphorylated activation loops namely at sites S177 and S181 while chain A is devoid of the same. A druggable site is located between the kinase domain (KD) and ubiquitin-like domain (ULD). It contains 3 α helixes belonging to KD and a single loop is present between 2 β sheets that belong to ULD (Liu et al. 2018a). The inhibitors targeted toward the ATP binding sites work irrespective of the active or inactive forms of IKKB as chain A and chain B possess similar KD regions. Interestingly, a pocket present at the KD-SDD surface is exposed in the B chain while in the A chain it is partially open which suggests the specific target region for selective antagonists (Napoleon et al. 2021).
The Zinc 15 library provides a comprehensive database of ligands useful for 3D molecular docking (Sterling and Irwin 2015). We performed molecular docking for IKKB with ligands from Zinc 15 FDA-approved library. The orthosteric IKKB binding site is lined by amino acids namely, Glu 97, Cys 99, Asp 166, Tyr 98, Gly 102, Met 96, Leu167, Asp 103, Leucine (Leu) 21, Valine (Val) 29, Val 152, Threonine (Thr) 23 and Isoleucine (Ile) 165, Ala 42 and Tyr 108 (Allegra et al. 2021; Leung et al. 2013; Xu et al. 2011). We obtained 3 dopamine receptor agonists namely apomorphine, rotigotine, and aripiprazole which showed good docking ability into the binding site of IKKB.
The apomorphine is a phenylethylamine with an ortho-catechol moiety attached to it. The OH group present in this moiety confers the ability to bind to dopamine receptors and also produce neuroprotective actions (Auffret et al. 2018; Csutoras et al. 2004). Surprisingly, the same OH group was involved in the binding to Glu 97 and Cys 99 with IKKB via hydrogen bonding as seen in Fig. 9. Hydrogen bonds primarily facilitate stronger protein and ligand interactions. It is known to improve the affinity of the complex through the displacement of water molecules bound to the proteins, into the neighboring solvent system (Ross et al. 2012). Therefore, the complexes need to have either a strong or weak H-bonding ability without which the interference from the water of the solvent system could hamper the affinity of the interactions (Ross et al. 2012). Our second molecule of interest, rotigotine, is a 5-hydroxytetraline-based selective D2, D3, D4, and D5 agonist (Wood et al. 2015). In addition to the hydrogen bonds, an additional salt bridge connecting Asp 103 with pyrrolidinium nitrogen was seen. Salt bridges are crucial to providing stability to the complexes at higher temperatures (Thomas and Elcock 2004) and therefore, they could confer the IKKB–rotigotine complex, a thermostable property. Another molecule, aripiprazole showed lesser XP G scores of − 7.778 Kcal/mol as compared to apomorphine and rotigotine which scored − 9.967 Kcal/mol and −9.039 Kcal/mol, respectively. Aripiprazole is a quinolinone-based partial D2 agonist (Hirose and Kikuchi 2005). It was able to predict a well-structured hydrophobic and positively charged pocket along the binding site of IKKB but could interact with only a single H-bond to predict a stable interaction. The oxygen atom of its quinoline-2-one moiety interacts with the Cys 99 amino acid via hydrogen bond which could provide it the desired stability. Therefore, we selected apomorphine, rotigotine, and aripiprazole for further studies.
The IFD predicts different confirmations upon binding of ligand to the receptor. Briefly, it involves steps like ligand docking with softened van der Waals radii, predicting side chains, minimization of protein and ligands, and rigorous XP docking (Allegra et al. 2021). The IFD analysis indicated that apomorphine predicted multiple docking poses with IKKB. The best pose was able to predict multiple hydrogen bonding along with an additional salt bridge that was missing in the rigid docking. The IFD of rotigotine and IKKB also showed improved interactions due to the presence of an additional hydrogen bond with pyrrolidinium nitrogen that was lacking in rigid docking. Surprisingly, Aripiprazole showed a greater number of interactions in IFD studies as compared to rigid docking. Along with hydrogen bonds and salt bridges, that promoted its stability, it also possessed Cl− halogen bonding. The halogen bonds like Cl− provide stability to the ligand and influence molecular folding via stabilization of the intermolecular interactions and intramolecular interactions (Metrangolo et al. 2008). These results chalk out the importance of IFD studies in understanding the best pose for the targets.
The MMGBSA is a preferred method to estimate the energy with which a ligand binds to proteins. Primarily, the binding free energy of the van der Waals energy, lipophilic energy, and coulomb energy is instrumental in the generation of the binding energy of the PL complexes. On the contrary, the binding free energy of the generalized Born electrostatic solvation energy and covalent binding energy have less effect on the binding energy scores (Jawarkar et al. 2022). This could be the reason why the binding free energy of IKKB–aripiprazole (−62.01 Kcal/mol) and IKKB–apomorphine (− 61.04 Kcal/mol) was higher in comparison to IKKB–rotigotine (− 49.95 Kcal/mol). A significantly higher DG bind H-bond, DG bind lipo, and DG bind vdW were seen in the IKKB–aripiprazole complex and apomorphine–IKKB complex as compared to rotigotine–IKKB.
The molecular dynamic studies of IKKB–apomorphine, IKKB–rotigotine, and IKKB–aripiprazole indicated the stability of the complexes through significant RMSD, timelines, and histograms. Similarly, the presence of good interactions was demonstrated with significant results in the histogram and timelines. This could suggest the desirable stability of apomorphine, rotigotine and aripiprazole in solvent system. The MMGBSA performed post-MD, indicated that these molecule complexes were relatively stable with less deviations under the influence of solvent system suggesting desirable binding free energies of the complexes.
Numerous human inflammatory diseases are thought to have their etiology influenced by dysregulated NF-kB signaling, the NF-kB signaling is a promising therapeutic area for the advancement of newer drugs. The IKKB protein kinase has historically been the target for drug discovery as a treatable regulator of canonical NF-kB signaling. Many chemicals that have been described as having activity against IKKB have shown efficacious results in pre-clinical inflammatory models. But systemic IKKB inhibition-related severe targeted toxicities and related safety concerns have so far precluded any IKKB inhibitors from receiving clinical approval (Gamble et al. 2012; Prescott and Cook 2018). So approved DA agonists including apomorphine, rotigotine and aripiprazole which could promote IKKB inhibition may be a better treatment strategy for neurological disorders.
Anglade et al. reported rotigotine showed anti-depressant activity and improved locomotor activity (Bertaina-Anglade et al. 2006). Interestingly, our literature search revealed multiple in vivo studies associated with the neuroprotective activity of apomorphine. Apomorphine attenuates the LPS-induced neuroinflammation and inhibits the inflammatory cytokines including IL-23 (Hara et al. 2017). Himeno et al. reported that apomorphine promotes Aβ degradation and enhances learning and memory seen in transgenic mice (Himeno et al. 2011) Continuous administration of apomorphine causes rapid dyskinesia, while on the contrary, rotigotine has been observed less motor complications including dyskinesia (Ye et al. 2013). D2R activation recruits β-Arrestin 2, which promotes interaction with Akt and creates a protein phosphatase 2A complex (Beaulieu et al. 2007). The β-Arrestin 2 elevated upon rotigotine administration and led to the formation of β-Arrestin 2–PP2A complex. The β-Arrestin 2 inhibits the phosphorylation of IkBα and inhibits NF-kB translocation into the nucleus and reduces caspase-1 stimulation as well as inhibits the NLRP3 inflammasome formation followed by decrease in the IL-1β and other proinflammatory cytokines (Yan et al. 2015; Yue et al. 2021) Nevertheless, rotigotine blocks the nuclear translocation of NF-kB p65 and lowers the luciferase activity of NF-kB. Rotigotine lowers the activation of NF-kB, and NOX-4 and reduces the secretion of reactive oxygen species (ROS) cytokines and chemokines including TNF-α, IL-8, Monocyte chemoattractant protein-1 (MCP-1) (Kang et al. 2021). Rotigotine-loaded microspheres reduce the production of ROS and inhibit the glial cell activation leading to a reduced inflammatory response followed by neuroprotection. Recently, Koch et al.conducted a clinical trial double-blind, placebo-controlled study, where rotigotine did not show significant enhancement in overall cognitive ability in AD patients, but it did improve frontal lobe-associated cognitive abilities and day to day activities (Koch et al. 2020). To affect as many deteriorating cells as feasible, simultaneously, a neuroprotective drug should either have a long half-life or be supplied constantly. There are still certain restrictions, though it is currently unclear if rotigotine has advantageous effects on dopamine receptors or not. The ability of rotigotine to activate each of the five different dopamine receptor types is also well documented. It is necessary to clarify the dopamine receptor subtype that might mediate the pharmacological activity.
As we attempt to elucidate, to the best of our ability, we understand the limitations involved in our study. The binding characteristics of rotigotine to IKKB, especially the physiological concentrations and affinity in silico could be estimated. Our study lacked the in vivo estimation of phosphorylated IKKB, various subunits of NF-kB related to canonical pathway neuroimaging studies, brain targeted formulations, which could provide a better idea about the neuroinflammation conditions.
Conclusion
The role of IKKB in mediating neuroinflammation is crucial in the conditions of neurodegeneration. We were able to predict the desirable binding ability of apomorphine, rotigotine and aripiprazole which are dopamine agonists predicted to inhibit IKKB in our study. This is the key to future in vivo and ex vivo studies. Yet, the further mechanism remains to be elucidated for which subsequent studies are warranted.
References
Allegra M, Tutone M, Tesoriere L, Attanzio A, Culletta G, Almerico AM (2021) Evaluation of the IKKβ binding of indicaxanthin by induced-fit docking, binding pose metadynamics, and molecular dynamics. Front Pharmacol 12:2400
Auffret M, Drapier S, Vérin M (2018) Pharmacological insights into the use of apomorphine in Parkinson’s disease: clinical relevance. Clin Drug Investig 38:287–312
Baby K, Maity S, Mehta CH, Suresh A, Nayak UY, Nayak Y (2021) Targeting SARS-CoV-2 main protease: a computational drug repurposing study. Arch Med Res 52:38–47
Basu Mallik S, Mudgal J, Hall S, Kinra M, Grant GD, Nampoothiri M, Anoopkumar-Dukie S, Arora D (2022) Remedial effects of caffeine against depressive-like behaviour in mice by modulation of neuroinflammation and BDNF. Nutr Neurosci 25:1836–1844
Beaulieu JM, Gainetdinov RR, Caron MG (2007) The Akt-GSK-3 signaling cascade in the actions of dopamine. Trends Pharmacol Sci 28:166–172
Bertaina-Anglade V, La Rochelle CD, Scheller DKA (2006) Antidepressant properties of rotigotine in experimental models of depression. Eur J Pharmacol 548:106–114
Chaiwut R, Kasinrerk W (2022) Very low concentration of lipopolysaccharide can induce the production of various cytokines and chemokines in human primary monocytes. BMC Res Notes 15:1–8
Csutoras C, Zhang A, Zhang K, Kula NS, Baldessarini RJ, Neumeyer JL (2004) Synthesis and neuropharmacological evaluation of R(-)-N-alkyl-11-hydroxynoraporphines and their esters. Bioorganic Med Chem 12:3553–3559
Frank MG, Weber MD, Watkins LR, Maier SF (2015) Stress sounds the alarmin: the role of the danger-associated molecular pattern HMGB1 in stress-induced neuroinflammatory priming. Brain Behavior Immunity 48:1–7
Gamble C, McIntosh K, Scott R, Ho KH, Plevin R, Paul A (2012) Inhibitory kappa B kinases as targets for pharmacological regulation. Br J Pharmacol 165:802–819
Golde T, Dickson D, Hutton M (2006) Filling the gaps in the Aβ Cascade hypothesis of Alzheimer's Disease. Curr Alzheimer Res 3:421–430
Gurram PC, Manandhar S, Satarker S, Mudgal J, Arora D, Nampoothiri M (2022) Dopaminergic signaling as a plausible modulator of astrocytic toll-like receptor 4: A crosstalk between neuroinflammation and cognition. CNS Neurol. Disord. Drug Targets, 21.
Halgren TA, Murphy RB, Friesner RA, Beard HS, Frye LL, Pollard WT, Banks JL (2004) Glide: a new approach for rapid, accurate docking and scoring. 2. enrichment factors in database screening. J Med Chem 47:1750–1759
Hara H, Kimoto D, Kajita M, Takada C, Kamiya T, Adachi T (2017) Apomorphine prevents LPS-induced IL-23 p19 mRNA expression via inhibition of JNK and ATF4 in HAPI cells. Eur J Pharmacol 795:108–114
Himeno E, Ohyagi Y, Ma L, Nakamura N, Miyoshi K, Sakae N, Motomura K, Soejima N, Yamasaki R, Hashimoto T et al (2011) Apomorphine treatment in Alzheimer mice promoting amyloid-β degradation. Ann Neurol 69:248–256
Hirose T, Kikuchi T (2005) Aripiprazole, a novel antipsychotic agent: Dopamine D2 receptor partial agonist. J Med Investig 52:284–290
Jawarkar RD, Sharma P, Jain N, Gandhi A, Mukerjee N, Al-Mutairi AA, Zaki MEA, Al-Hussain SA, Samad A, Masand VH et al (2022) QSAR, Molecular Docking, MD Simulation and MMGBSA calculations approaches to recognize concealed pharmacophoric features requisite for the optimization of ALK tyrosine kinase inhibitors as anticancer leads. Molecules 27:4951
Ju Hwang C, Choi D-Y, Park MH, Hong JT (2017) NF-κB as a key mediator of brain inflammation in Alzheimer’s Disease. CNS Neurol Disord Drug Targets 18:3–10
Kang H, Yu H, Fan J, Cao G (2021) Rotigotine protects against oxidized low-density lipoprotein(ox-LDL)-induced damages in human umbilical vein endothelial cells(HUVECs). Bioengineered 12:10568–10579
Kinra M, Joseph A, Nampoothiri M, Arora D, Mudgal J (2021) Inhibition of NLRP3-inflammasome mediated IL-1β release by phenylpropanoic acid derivatives: in-silico and in-vitro approach. Eur J Pharm Sci, 157.
Koch G, Motta C, Bonnì S, Pellicciari MC, Picazio S, Casula EP, Maiella M, Di Lorenzo F, Ponzo V, Ferrari C, et al (2020) Effect of rotigotine vs placebo on cognitive functions among patients with mild to moderate Alzheimer disease: a randomized clinical trial. JAMA Netw Open, 3.
Krueger J (2008) The role of cytokines in sleep regulation. Curr Pharm Des 14:3408–3416
Leung CH, Chan DSH, Li YW, Fong WF, Ma DL (2013) Hit identification of IKKβ natural product inhibitor. BMC Pharmacol Toxicol, 14.
Li J, Abel R, Zhu K, Cao Y, Zhao S, Friesner RA (2011) The VSGB 2.0 model: a next generation energy model for high resolution protein structure modeling. Proteins Struct Funct Bioinforma 79:2794–2812
Li M, Zhang C, Zhou L, Sun X, Wang T, Fu F (2022) Continuous activation of dopamine receptors alleviates LPS-induced liver injury in mice via β-arrestin2 dependent Akt/NF-κB pathway. Front Pharmacol 13:710
Liu H, Liang H, Meng H, Deng X, Zhang X, Lai L (2018a) A novel allosteric inhibitor that prevents IKKβ activation. Medchemcomm 9:239–243
Liu Q, Li Y, Liu Y, Zhao Y, Li X, Zhang Y, Wang C, Huang W, Wang X (2018b) A dopamine D1 receptor agonist improved learning and memory in morphine-treated rats. Neurol Res 40:1080–1087
Madhavi Sastry G, Adzhigirey M, Day T, Annabhimoju R, Sherman W (2013) Protein and ligand preparation: parameters, protocols, and influence on virtual screening enrichments. J Comput Aided Mol Des 27:221–234
Metrangolo P, Meyer F, Pilati T, Resnati G, Terraneo G (2008) Halogen bonding in supramolecular chemistry. Angew Chemie - Int Ed 47:6114–6127
Millan MJ, Dekeyne A, Gobert A, Brocco M, Mannoury la Cour C, Ortuno JC, Watson D, Fone KCF (2020) Dual-acting agents for improving cognition and real-world function in Alzheimer’s disease: Focus on 5-HT6 and D3 receptors as hubs. Neuropharmacology, 177.
Mudgal J, Basu Mallik S, Nampoothiri M, Kinra M, Hall S, Grant GD, Anoopkumar-Dukie S, Davey AK, Rao CM, Arora D (2020) Effect of coffee constituents, caffeine and caffeic acid on anxiety and lipopolysaccharide-induced sickness behavior in mice. J Funct Foods 64:103638
Napoleon JV, Singh S, Rana S, Bendjennat M, Kumar V, Kizhake S, Palermo NY, Ouellette MM, Huxford T, Natarajan A (2021) Small molecule binding to inhibitor of nuclear factor kappa-B kinase subunit beta in an ATP non-competitive manner. Chem Commun 57:4678–4681
Navakkode S, Chew KCM, Tay SJN, Lin Q, Behnisch T, Soong TW (2017) Bidirectional modulation of hippocampal synaptic plasticity by Dopaminergic D4-receptors in the CA1 area of hippocampus. Sci Rep, 7.
Park SM, Chen M, Schmerberg CM, Dulman RS, Rodriguiz RM, Caron MG, Jin J, Wetsel WC (2016) Effects of β-arrestin-biased Dopamine D2 receptor ligands on schizophrenia-like behavior in hypoglutamatergic mice. Neuropsychopharmacology 41:704–715
Prescott JA, Cook SJ (2018) Targeting IKKβ in cancer: challenges and opportunities for the therapeutic utilisation of IKKβ inhibitors. Cells, 7.
Ray R, Birangal SR, Fathima F, Bhat GV, Rao M, Shenoy GG (2022) Repurposing of approved drugs and nutraceuticals to identify potential inhibitors of SARS-COV-2’s entry into human host cells: a structural analysis using induced-fit docking, MMGBSA and molecular dynamics simulation approach. Mol Simul 48:367–386
Ross GA, Morris GM, Biggin PC (2012) Rapid and accurate prediction and scoring of water molecules in protein binding sites. PLoS ONE 7:1–13
Sankhe R, Rathi E, Manandhar S, Kumar A, Pai SRK, Kini SG, Kishore A (2021) Repurposing of existing FDA approved drugs for Neprilysin inhibition: an in-silico study. J Mol Struct 1224:129073
Satarker S, Bojja SL, Gurram PC, Mudgal J, Arora D, Nampoothiri M (2022a) Astrocytic Glutamatergic Transmission and Its Implications in Neurodegenerative Disorders. Cells 11:1139
Satarker S, Maity S, Mudgal J, Nampoothiri M (2022b) In silico screening of neurokinin receptor antagonists as a therapeutic strategy for neuroinflammation in Alzheimer’s disease. Mol Divers 26:443–466
Sterling T, Irwin JJ (2015) ZINC 15-Ligand discovery for everyone. J Chem Inf Model 55:2324–2337
Thomas AS, Elcock AH (2004) Molecular simulations suggest protein salt bridges are uniquely suited to life at high temperatures. J Am Chem Soc 126:2208–2214
Veerappan K, Natarajan S, Ethiraj P, Vetrivel U, Samuel S (2017) Inhibition of IKKβ by celastrol and its analogues - an in silico and in vitro approach. Pharm Biol 55:368–373
Wood M, Dubois V, Scheller D, Gillard M (2015) Rotigotine is a potent agonist at dopamine D1 receptors as well as at dopamine D2 and D3 receptors. Br J Pharmacol 172:1124–1135
Wu Y, Hu Y, Wang B, Li S, Ma C, Liu X, Moynagh PN, Zhou J, Yang S (2020) Dopamine Uses the DRD5-ARRB2-PP2A Signaling Axis to Block the TRAF6-Mediated NF-κB Pathway and Suppress Systemic Inflammation. Mol Cell 78:42-56.e6
Xu G, Lo YC, Li Q, Napolitano G, Wu X, Jiang X, Dreano M, Karin M, Wu H (2011) Crystal structure of inhibitor of κb kinase β. Nature 472:325–330
Yan Y, Jiang W, Liu L, Wang X, Ding C, Tian Z, Zhou R (2015) Dopamine controls systemic inflammation through inhibition of NLRP3 inflammasome. Cell 160:62–73
Ye L, Guan X, Tian J, Zhang J, Du G, Yu X, Yu P, Cen X, Liu W, Li Y (2013) Three-month subchronic intramuscular toxicity study of rotigotine-loaded microspheres in SD rats. Food Chem Toxicol 56:81–92
Yue S, Wang T, Yang Y, Fan Y, Zhou L, Li M, Fu F (2021) Lipopolysaccharide/D-galactosamine-induced acute liver injury could be attenuated by dopamine receptor agonist rotigotine via regulating NF-κB signaling pathway. Int Immunopharmacol, 96.
Zhang F, Qian L, Flood PM, Shi JS, Hong JS, Gao HM (2010) Inhibition of IκB kinase-β protects dopamine neurons against lipopolysaccharide-induced neurotoxicity. J Pharmacol Exp Ther 333:822–833
Acknowledgements
The authors would like to thank the Manipal-Schrodinger Center for Molecular Simulations Lab for providing the required facilities to carry out this research. Our deepest gratitude toward Dr. Gopalan Kutty, Dr. Sreedhara Ranganath Pai, Dr. Yogendra Nayak, and Mr. Krishna Prasad Baby for their untiring support. All India Council for Technical Education-Quality Improvement Programme (AICTE-QIP) fellowship to Prasada Chowdari Gurram is truly acknowledged.
Funding
Open access funding provided by Manipal Academy of Higher Education, Manipal. This research did not receive any specific grant from funding agencies in the public, commercial and nonprofit sectors.
Author information
Authors and Affiliations
Contributions
PCG contributed to data collection, methodology, and writing; SS contributed to draft preparation and software management; AN curated the data, JM contributed to reviewing and editing, MN contributed to conceptualization, visualization, and investigation.
Corresponding author
Ethics declarations
Conflict of interest
The authors report there is no competing interest to declare.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Gurram, P.C., Satarker, S., Nassar, A. et al. Virtual structure-based docking and molecular dynamics of FDA-approved drugs for the identification of potential IKKB inhibitors possessing dopaminergic activity in Alzheimer’s disease. Chem. Pap. 77, 1971–1988 (2023). https://doi.org/10.1007/s11696-022-02598-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11696-022-02598-y