Abstract
The evaluation of the bioavailability of topically applied medications that act inside or under the skin is a challenging task. Herein, the current study describes a simple, quick, and low-cost electrochemical platform for determining butenafine hydrochloride (BTH) that is mainly prescribed as a treatment of dermatophytosis, applying titanium nanoparticles and an ionic liquid as outstanding mediators. In terms of low detection limits (61.63 nM) and extensive range of 2.21 × 10–7–13.46 × 10–5 M, the established electrochemical technique provided worthy analytical performance for butenafine hydrochloride sensing. The suggested sensor's practical applicability was effectively demonstrated in pharmaceutical preparations, actual stratum corneum samples, and simultaneous detection of butenafine hydrochloride and Itraconazole in pharmaceutical preparation for the first time. All of the experimental factors, like the pH and scan rate, have been investigated and optimized. Diffusion coefficients of butenafine hydrochloride at bare and modified sensors were calculated.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Butenafine hydrochloride (BTH) is a benzylamine antifungal drug with great efficacy of treatment and a long duration of action when administered topically against various mycoses (Chiou et al. 2021). The severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) proteolytic activity has been discovered to be blocked by BTH (Chiou et al. 2021). At doses less than 10 μM, butenafine inhibited SARS-CoV-2 activity by 50% (Chiou et al. 2021). BTH has the chemical name N-(4-tert-butylbenzyl)-N-methyl-naphthalenemethylamine hydrochloride (Nahm et al. 1999). BTH, similar to the allylamine medications, inhibits squalene epoxidase (a sterol synthesis enzyme, which transforms squalene to ergosterol), causing squalene to accumulate intracellularly. Fungal cell membranes are disrupted because of this accumulation. Besides, BTH possesses an anti-inflammatory property in vivo. After UVB treatment, it minimizes cutaneous erythema (Nahm et al. 1999).
In the soil and other parts of the environment, there are many distinct forms of fungal germs. They can act directly on the surface of the body, infecting the lips, nails, and skin, as well as causing a fungal infection. The use of nanocarriers as a topical therapy for fungal infections has shown to be a feasible delivery strategy (Mahdi et al. 2021).
BTH demonstrates a high level of fungicidal activity, particularly against dermatophytes, aspergilli, dimorphic, and dematiaceous fungi. Following topical administration, the medication exhibits great penetration into the epidermis and a lengthy retention duration, imparting residual therapeutic efficacy after treatment discontinuation. For tinea pedis, tinea corporis, and tinea cruris, topical butenafine 1% cream has been shown to be effective for short-term treatment (Sakagami 2010).
To best of our knowledge, different analytical techniques were used to determine BTH including HPLC (Ankam et al. 2009; Bhosale and Rajput 2011; Miron et al. 2014), LC (Barth et al. 2011a, 2011b; Song et al. 2011), and potentiometric methods (Ali et al. 2020; Frag etal. 2020).
Itraconazole (ITZ) is an antifungal medication taken orally that inhibits cytochrome P450-dependent enzymes, causing ergosterol production in fungal cell membranes to be inhibited (Feng 2003). ITZ is used in combination with BTH cream to treat pityriasis versicolor and reduce the long-term recurrence rate without causing any noticeable side effects (Hong-Xiao et al. 2010). Simultaneous determination of ITZ and BTH is therefore regarded as a new and promising field of research.
Compared to other techniques, electrochemical methods are advantageous over other options since they allow for faster, cheaper, and cleaner analysis as well as high sensitivity (Mohamed et al. 2021, 2020). Analytical methods that use chemically modified electrodes to detect minute concentrations of physiologically relevant substances have long been considered very sensitive and specific (Mohamed 2018; Mohamed et al. 2016; Saadeh et al. 2018; Ghalwa et al. 2020).
In recent years, nanoparticles have gained a lot of attention because of their use in biosensing technology for the detection of different pharmaceuticals. Electrochemical sensing processes have been shown to be useful in the detection of toxins, environmental management, and food safety (Mohamed 2018; Ghoniem et al. 2021).
Because of its high aspect ratio, optical transparency, exceptional biocompatibility, and relatively robust conductivity, titanium dioxide nanoparticles have been frequently employed in voltammetry for the research of various analytes (Sanghavi and Srivastava 2013; Mohamed et al. 2017a).
Ionic liquids have lately attracted a lot of attention in a variety of fields, notably electrochemistry, high viscosity and nonvolatility, as well as strong ionic conductivity, thermal stability, oxidative and thermal stability, huge solvation power, and non-flammability are some of its distinctive qualities (Mohamed et al. 2019).
In the present work, we first report the determination of BTH in a drug substance and pharmaceutical preparation using Ti nanoparticles and IL. We also present some new insights into the attractive square wave voltammetry (SWV) of this electrode for the simultaneous determination of BTH and ITZ.
Experimental
Instrumentation
The electrochemical characteristics of the samples were analyzed using the Bio-logic SP 150 electrochemical workstation. A C3-stand from BASi research products (USA) was used to connect a one-compartment cell with a three-electrode setup to the electrochemical workstation. As an auxiliary electrode, a platinum wire, while carbon paste electrode (CPE) was used as working electrode, and Ag/AgCl (3.0 M NaCl) was used as reference electrode. All electrodes were obtained from BASi research products (USA).
For pH measurements, a Cyberscan 500 digital (EUTECH Instruments, USA) pH-meter with a glass combination electrode was used.
Electrochemical impedance spectroscopy measurements were performed over a frequency range of 100 mHz to 100 kHz. All electrochemical experiments were carried out at a temperature of 25 °C.
Materials and reagents
Western Pharmaceutical Industries generously provided BTH (B.N. 83160221). According to the manufacturer's certificate of analysis (COA), the BTH assay was 99.50 percent accurate (HPLC). 1-Hexyl-3-methylimidazolium hexafluorophosphate (IL) was obtained from Sigma-Aldrich. Butaximark® was obtained from the market and includes 10.00 mg BTH/1.00 gm.
Aldrich provided graphite powder with a particle size of 50.0 μm. For the manufacture of the paste electrodes, paraffin oil (Merck Co. Germany) was employed as the pasting liquid. As a supporting electrolyte, 0.04 M Britton–Robinson buffer (B–R buffer) was used. To achieve the necessary pH, phosphoric, acetic, and boric acids (Sigma-Aldrich) were combined with 0.20 M NaOH (2.0–6.0). All of the solutions were made with arium® pro, sartorius (Goettingen, Germany) ultra-pure water.
Preparation of different electrodes
Using a pestle and mortar, graphite powder (0.50 g) and paraffin oil (0.30 mL) were mixed to make a carbon paste electrode (CPE). To get a shining look, the carbon paste was packed into the hole of an electrode body, scraped out the excess with a typical paper, and the electrode was polished on a smooth paper.
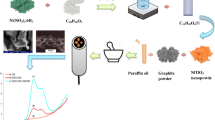
A mixture of 985.00 mg graphite powder, 15.0 mg Titanium nanoparticles (Ti), and the 5 μL ionic liquid was used to make the improved sensor.
Using a mortar, the mixture was gently homogenized for 45.0 min. The paste was then made by adding 0.60 mL of paraffin oil. The paste was loaded into the electrode's hole and smoothed out with filter paper until it was gleaming. By removing the surplus paste from the syringe and republishing it with weighing paper, a new surface was obtained.
Ti-IL/CPE was the name given to the modified electrode. Various modified carbon pastes containing various percentages of Ti nanoparticles (0.5, 1.0, 1.5, and 2.0 percent w/w) were made in the same way.
Standard and working solutions
A stock solution of BTH (1.0 × 10–2 M) was prepared by dissolving 353.93 mg in ethanol HPLC-grade (Sigma-Aldrich) in a 100.0-mL volumetric flask.
Working solutions for BTH were made by diluting the stock standard solution with ethanol to obtain solutions in the concentration range of 1.0 × 10−3 to 1.0 × 10−4 M. At 4 °C, these solutions remained stable for roughly a week.
Experimental procedure
The modified electrode Ti-IL/CPE was cycled between 0.0 and 1400.0 mV with a scan rate of 100.0 mV s−1 in 0.04 M B–R buffer solution of pH 2.0 numerous times before any voltammetric measurements were taken until a repeatable response was attained. The modified Ti-IL/CPE electrode was then placed in a new cell containing 0.04 M B–R buffer at pH 2.0 and the appropriate concentrations of BTH. With a scan rate of 100.0 mV s−1, cyclic voltammograms (CV) were recorded between 0.0 and 1400.0 mV.
The recommended procedure for calibration curves
Using a micropipette, aliquots of BTH ranging from 2.21 × 10–7 to 13.46 × 10–5 M were put into a series of 5-mL volumetric flasks, and the volume was filled to the mark with 0.04 M B–R buffer pH 2.0. After transferring the solution to the electrolytic cell, a square wave voltammogram was obtained. The SWV analytical process for determining BTH was verified according to the International Conference on Harmonization (ICH) principles (Zhang et al. 2020).
Application to pharmaceutical formulations
A sufficient amount of Butaximark® cream equivalent to a stock solution of concentration 1.0 × 10−3 M of BTH was weighed, transported to a 100-mL calibrated flask, and dissolved in 50 mL of ethanol.
The solution was sonicated at 40 °C for 45 min, then allowed to cool to ambient temperature before being filtered through 0.45 μm filter paper. 20 µL was accurately transferred to a 5.0-mL volumetric flask, which was then filled to the mark with 0.04 M B–R buffer pH 2.0.
The concentrations of the cited medication were estimated using the recommended approach listed under "Recommended procedure for calibration curves." The accuracy of the proposed method was assured by the application of the standard addition technique.
Application to real samples
Tape stripping
One healthy female volunteer (30 years of age), with no history of dermatological diseases, was chosen for this study. She was informed not to apply any cosmetic treatment to the test area on her forearm 24 h prior to the start of the study. 30 min before the experiment, the volar aspect of the forearm of each subject was cleaned. Using adhesive labels, the application site was marked on the forearm keeping one site as a blank (dimensions of each area 2 × 5 cm2) (Abdel-Salam et al. 2017).
Approximately, 5 mg/cm2 of 1% Butaximark® cream was applied uniformly on the selected site by means of a glass rod (Herkenne et al. 2008). After 12 h of application, the residual formulation was removed by gentle wiping with cotton swab (Parfitt et al. 2011).
The TS procedure was performed utilizing 15 adhesive tape strip at each site (Au et al. 2010) using Scotch TM tape (No. 845 Book Tape 3 M company, St. Paul, MN). The tape was pressed with a 100 g roller applied back and forth for 10 times to remove any furrows and wrinkles on the TS procedures (Teichmann et al. 2007). The tapes were removed with a rapid movement, changing the direction of the removal of each strip in order of clockwise rotation. Scheme 1 displays the steps of the tape-stripping method.
Steps of the tape stripping method: A marking the sites using adhesive labels, B application of the cream on the marked skin areas, C homogeneous distribution of the cream, D adhesive tapes are applied after each duration, E adhesive tape are pressed with a roller on the skin and F stripping of the tape
Collecting samples
The 15 strips were combined and extracted over 16 h in 10 ml ethanol. The obtained extract was filtered through 0.2 μm pore size syringe filter. 0.5 ml of the filtrate was accurately transferred to a 5.0-mL volumetric flask and the volume was completed to the mark with 0.04 M B–R buffer pH 2.0. The recommended procedure mentioned under," Recommended procedure for calibration curves" was followed and the concentrations of the cited drug were calculated. The accuracy of the proposed method was assured by the application of standard addition technique.
Results and discussion
Characterization using SEM
In order to characterize the surface morphology of the electrodes, scanning electron microscopy (SEM) is considered a critical approach to getting vital information about the electrode surface morphology (Mohd Norsham et al. 2020).
Figure 1 shows how scanning electron microscopy (SEM) data were applied to compare and define the morphological properties of various electrodes. Large graphite flakes with disorganized distribution and irregular form dominated the bare Ti/CPE surface. The gaps between the isolated graphite and Ti particles were relatively big, affecting the charges transmitted in the vertical direction of planes. While the separate carbon layers vanished from the surface of the Ti-IL/CPE, a homogeneous surface with many consistently micrometer-sized particles developed.
Using Randles–Sevcik (Allen and Larry 2001), the surface area of the constructed sensors was measured by acquiring CVs with 5.0 mM K3Fe (CN)6 probe at various scans onto CPE and 1.50% (w/w) Ti-IL/CPE (Fig. 2). The surface areas obtained for CPE and 1.50% (w/w) Ti-IL/CPE were 0.056 and 0.257 cm2, respectively. The obtained facts showed that the combination of Ti nanoparticles and ionic liquid leads to increased electrode area and electrochemical activity in Ti-IL/CPE sensor (Mohamed et al. 2019, 2017b).
Effect of Titanium nanoparticles content
The proportion of Ti nanoparticles used in sensor manufacture affects Ti-IL/CPE electrochemical behavior. As a function of Ti nanoparticles, the anodic peak current for BTH oxidation at Ti-IL/CPE electrode was investigated using the ratios from 0.5 to 2.0%. The optimal anodic peak current was obtained when the CPE was produced with 1.50% Ti nanoparticles. The background current was raised by increasing the number of Ti nanoparticles, resulting in poor reproducibility of the BTH response (Figure S1). The increase in background current is referred to the arising of non-Faradaic current. Experimental settings in fundamental voltammetric research are usually constructed so that non-faradaic contributions are quite minimal (Barrosse-Antle et al. 2010).
Ti-IL/CPE electrochemical behavior
The CVs of unmodified CPE, Ti/CPE, IL/CPE and Ti-IL/CPE were examined using 5.0 mM K3Fe(CN)6 as the electrochemical probe in order to better understand the electrochemical behavior of both electrodes. Figure 3A shows that when compared to the unmodified sensor, the highest Ip was obtained for 1.50% (w/w) Ti-IL/CPE, which may be ascribed to its greater surface area and good electrochemical conductivity owing to the integration of Ti nanoparticles and IL. Ti-IL/CPE has 2.97 times more redox peaks than CPE.
Furthermore, CV responses in the presence of 0.10 mM BTH in 0.04 M B–R buffer were used to analyze the modified sensor's electrochemical behavior (pH 2.0). The electrochemical oxidation of BTH is represented by the anodic peak current (Fig. 3B) was 14.10 µA at 1187.0 mV when using unmodified CPE, whereas the current magnitude of BTH increased to 40.69 A at 1103.0 mV when using 1.5% Ti-IL/CPE. Comparing the electrodes responses, the Ti-IL/CPE has the highest anodic current. As a result of their strong catalytic activity, Ti nanoparticles act as a mediator, increasing the rate of electron transfer.
pH optimization
Cyclic voltammograms were carried out on the Ti-IL/CPE using 0.1 mM BTH in a pH range of 2 to 6 of 4.0 × 10–2 M buffer solution.
The behavior of BTH at different pH levels is depicted in Fig. 4 as the peak potentials (Ep) shift to a less positive potential as the value of the pH rises and the current decreases until the peak vanishes and the clear solution becomes turbid. At pH 6.0, the peak vanishes and the clear solution becomes turbid. At pH 2.0, the greatest anodic peak current (Ip) was measured, and this pH was chosen as the best for the selective and sensitive measurement of BTH.
The voltammetric responses show a linear relationship with the buffer solution pH, which can be fit as Ep (V) = 1.204–0.053 pH; R = 0.9996. A well-developed anodic peak current was observed spanning the pH range of 2.0 to 5.0, as shown in (Fig. 4).
Furthermore, when the pH is raised to 5.0, the oxidation peak shifts to a lower positive potential, representing that the oxidation process is irreversible, as validated by CV analysis. According to the Nernst equation, the slope of the linear peak potential and pH correlation for BTH is 53.0 mV/pH, showing that the electrochemical oxidation process comprises the same number of electrons and protons transfer reactions. The proposed mechanism for the oxidation of BTH is revealed in Scheme 2.
Scan rate impact
To explore the process behavior that was occurring at the sensor surface and to evaluate the electrochemical strategy of the sensor reaction, CVs of a constant concentration (0.10 mM BTH) at pH 2.0 were collected at varied scan rates (Figure S2).
The increase in scan rate was accompanied by an increase in currents (Ip). As shown in Figure S2, inset A, the Ip increased linearly with the scan rate square root, indicating that the process is diffusion regulated. For the oxidation process, the appropriate linear equation was Ip (µA) = 3.28 v1/2 (Vs−1)1/2 + 5.56; R = 0.9995. The processes prevalent at the working electrode surface are clearly diffusion-controlled, based on the foregoing findings.
As illustrated in Figure S2, inset B, direct proportionality was found between log current and log scan rate in the 20–300 mVs−1 range using the formula: log I (A) = 0.7422 + 0.4235 log v; R = 0.9994. The slope of the derived linear relationship is less than 0.5 Ti-IL/CPE, implying that the electroactive constituents are transmitted via a diffusion-controlled course, implying that the modified electrode plays a substantial role (Laviron et al. 1980).
Chronoamperometric measurements
Cottrell's law (Yarin et al. 2014) describes the current corresponding to the electrochemical reaction (under diffusion control) for an electroactive material with the diffusion coefficient (D) as the following:
where A is the electrochemically active area, D is the diffusion coefficient, C * is the bulk concentration of ferrocyanide, while the rest of the parameters have their standard definitions.
The diffusion coefficient was calculated for unmodified and Ti-IL/CPE using the Cottrell equation (Yarin et al. 2014), Figure S3. The diffusion coefficient values were found to be 8.65 × 10–6 cm2 s−1 and 5.96 × 10–5 cm2 s−1for unmodified and Ti-IL sensors, respectively. The Ti-IL/CPE diffusion coefficient is greater than that of CPE, showing that the modified electrode has more diffusion capacity than the unmodified electrode. The Ti nanoparticles and ionic liquid facilitated the charge transfer and diffusion of ions through the electrode surface.
Validation of the proposed Ti-IL/CPE
The linearity, selectivity, precision, and accuracy of the created electrochemical platform were tested in accordance with the International Conference on Harmonization (ICH) requirements (Zhang et al. 2020).
Linearity
The BTH determination was used to validate the Ti-IL/CPE sensor that had been developed. Using the Ti-IL/CPE, it was discovered that the peak currents related to the electrochemical oxidation of BTH are linearly subordinate on BTH quantity across the range of 2.21 × 10–7–13.46 × 10–5 M, while the regression equation is found to be Ip (µA) = 0.371C + 3.03; R2 = 0.9998, Fig. 5. Table S1 shows that the suggested platform has a high level of accuracy and precision.
A The SWV using Ti-IL/CPE sensor in pH 2.0 B–R buffer corresponding to: 2.21 × 10–7–13.46 × 10–5 M BTH (v = 100 mV s−1), PH = 0.25 V, PW = 0.50 V, SH = 0.02 V. B) SWV for ITZ in the concentration range 6.62 × 10–6–1.00 × 10–4 M using Ti-IL/CPE (v = 100 mV s−1), PH = 0.25 V, PW = 0.50 V, SH = 0.02 V. The inserts are current vs. concentration plots
On the other hand, the linearity range for ITZ found to be 6.62 × 10–6 M to 1.00 × 10–4 M and the regression equation is found to be Ip (µA) = 0.1161C + 1.813; R2 = 0.9987.
Detection and quantitation limits
The limit of detection (LOD) and the limit of quantification (LOQ) were determined to be 6.163 × 10–8 and 2.054 × 10–7 M, respectively, Table S1. By utilizing the formulas 10S/b and 3.3S/b (where S is defined as the standard deviation of the Ip and b is referring to the calibration curve slope), it is possible to calculate the calibration curve slope for both parameters.
Accuracy
The percent recovery of the proposed electrochemical procedure was used to assess accuracy (Zhang et al. 2020). Five repeat assays for varying concentrations of BTH in the quantification range were performed. The accuracy of the disclosed technique for the assay of BTH was demonstrated with satisfactory results. Compared to previous related methodologies, the proposed approach for evaluating the studied drug in drug substances and medical products showed statistically significant findings. In accordance with the results of Table S2, the calculated t- and F-test values were lower than the reported values, suggesting that there was no statistically significant difference in accuracy and precision.
Repeatability and intermediate precision
The mean RSD % (n = 3) was calculated after triplicate examination of three concentrations of BTH (10.98, 19.61, and 32.25 µM) on the same day (intraday test) or three successive days (interday assay). As shown in Table S3, the estimated average RSD% for the intra- and inter-day studies did not exceed 1%, demonstrating acceptable accuracy. The stability of the Ti-IL/CPE was investigated throughout an eight-day storage period in the air with no change in the voltammetric peak current. Table S3 shows that the sensor maintained 98.87–99.02 percent of its initial response after 14 days.
Interference studies
Because of the existence of ascorbic acid (AA) and uric acid (UA) in a variety of real samples, the electrochemical oxidation of BTH employing Ti-IL/CPE through SWVs was also examined (Ghoniem et al. 2021). Figure S4 shows how remote these are from the analytical peak of interest. Using a given drug concentration, the influence of a variety of materials that might potentially affect the electroanalytical signals of BTH was also investigated (1.0 × 10–5 M). Each of these drugs was added to a fixed BTH amount using the optimum pH solution, and the magnitude of the BTH signal was measured. None of the potential interfering compounds tested interfered with the electroanalytical detection of BTH. Each interfering material had a tolerance limit of less than 5.0%. Furthermore, it was shown that 100-time increased amounts of inorganic ions (e.g., sodium, potassium, calcium, zinc, magnesium, iron, Cl−, SO42−, and NO3−) had no impact on oxidation peak currents.
Analytical application of Ti-IL/CPE
Application to the medicinal formulation
The proposed technique had been tested on a commercially available medicinal cream. Table 1 displays the results. The recommended sensor's accuracy is demonstrated by the recovery figures. According to the findings of the experiments, this sensor is extremely sensitive to identifying lower and higher BTH concentrations in the dosage form.
Application to real samples
As one of the advantages of topical application is that the infected area can be treated with minimum systemic toxicity but the undetectable systemic levels cause difficulty in measuring the systemic availability of the drug (Alberti et al. 2001). Therefore, to evaluate topical drug bioavailability and the effectiveness of the applied cream, the convenient metric is measuring drug concentration in the stratum corneum (SC), as antifungals targeting this organ. This is done by quantifying BTH during the sequential tape stripping process after treatment with Butaximark® cream on human subjects by SWV.
Tape stripping application
The concentration of BTH in the SC tissue subsequent a single topical claim of 5 mg/cm2 of Butaximark® to a volunteer was determined using SWVs and it was found to be around 5.26 µM/cm2 (average of three determinations). The results are displayed in Table 2. This relevant concentration is above the minimum inhibition concentration (MIC) for BTH, which should in clinical use result in effective inhibition of the growth of fungi (Syed and Maibach 2000; Kokjohn et al. 2003). Standard addition technique was applied to eliminate any matrix effect of the proposed method. As shown in Table 2, the mean recovery% indicates that BTH can be successfully determined in SC tissue after the topical application of the cream.
Application to the simultaneous determination of butenafine hydrochloride and Itraconazole
According to the literature, no study has been published that uses electrochemical techniques to simultaneously determine BTH and Itraconazole (ITZ). The detection of BTH and ITZ in pharmacological compounds concurrently utilizing the Ti-IL/CPE technology is thus a significant element of our research.
Ti-IL/CPE was used to record the SWV, while the immediate levels of BTH and ITZ were changed. In the SWVs, anodic peaks with well-defined potentials of 1035.69 and 651.27 mV were observed, which corresponded to the oxidation of BTH and ITZ, respectively, proving that the simultaneous detection of both medicines is attainable at the Ti-IL/CPE, as revealed in Fig. 6. The calibration graphs were linearly associated to BTH and ITZ through the range 7.65 × 10−6–9.091 × 10−5 M. We calculated the regression equations and concluded that they were as follows:
The value of BHT sensitivity in case of the single determination (0.3671 μA/µM) is extremely close to that observed in the case of a combination (0.3759 μA/µM) including BTH and ITZ. According to the results, BTH and ITZ oxidation are independent, and concurrent assessment of the combination of BTH and ITZ is accurate and selective.
Comparing to the previous electrochemical potentiometric determination of BTH, the present voltammetric work showed the lowest LOD and the wider dynamic range, Table 3.
Conclusion
The measurement of BTH in drug substance, pharmaceutical preparations, and real samples were done using a carbon paste electrode enhanced with Ti nanoparticles and ionic liquid in this work. The voltammetric responses of the CV and SWV tests revealed effective electrochemical activity, high sensitivity, selectivity, and repeatability. The technique was used to measure BTH in drug substance, drug products, and real-life samples. The suggested method's low detection limit, along with the convenience of electrode fabrication, make it ideal for reliable drug determination in regular quality control work, and the findings demonstrate that the proposed sensor is capable of investigating the dermatopharmacokinetics of BTH.
References
Abdel-Salam FS, Mahmoud AA, Ammar HO, Elkheshen SA (2017) Nanostructured lipid carriers as semisolid topical delivery formulations for diflucortolone valerate. J Liposome Res 27:41–55
Alberti I, Kalia YN, Naik A, Bonny J-D, Guy RH (2001) In vivo assessment of enhanced topical delivery of terbinafine to human stratum corneum. J Control Release 71:319–327
Ali TA, Mohamed GG, Abdeen D, El-Dessouky H (2020) Highly sensitive electrochemical sensor determination of drug in pharmaceutical analysis. Egypt J Chem 63:8–10
Allen JB, Larry RF (2001) Electrochemical methods fundamentals and applications, John Wiley & Sons
Ankam R, Mukkanti K, Durgaprasad S, Khan M (2009) Simultaneous HPLC determination of butenafine hydrochloride and betamethasone in a cream formulation. Indian J Pharm Sci 71:547
Au WL, Skinner MF, Kanfer I (2010) Comparison of tape stripping with the human skin blanching assay for the bioequivalence assessment of topical clobetasol propionate formulations. J Pharm Pharm Sci 13:11–20
Barrosse-Antle LE, Bond AM, Compton RG, O’Mahony AM, Rogers EI, Silvester DS (2010) Voltammetry in room temperature ionic liquids: comparisons and contrasts with conventional electrochemical solvents. Chem–asian J 5:202–230
Barth AB, Pereira RL, de Vargas BA, Volpato NM (2011a) A simple and rapid method to assess butenafine hydrochloride in skin samples and a comparative cutaneous retention study of two marketed formulations. Biomed Chromatogr 25:1132–1137
Barth AB, De Oliveira GB, Malesuik MD, Paim CS, Volpato NM (2011b) Stability-indicating LC assay for butenafine hydrochloride in creams using an experimental design for robustness evaluation and photodegradation kinetics study. J Chromatogr Sci 49:512–518
Bhosale SD, Rajput SJ (2011) RP-HPLC method for simultaneous determination of butenafine hydrochloride and betamethasone dipropionate in a cream formulation. J AOAC Int 94:106–109
Chiou W-C, Hsu M-S, Chen Y-T, Yang J-M, Tsay Y-G, Huang H-C, Huang C (2021) Repurposing existing drugs: identification of SARS-CoV-2 3C-like protease inhibitors. J Enzym Inhib Med Chem 36:147–153
Feng J (2003) Stretching of a straight electrically charged viscoelastic jet. J Nonnewton Fluid Mech 116:55–70
Frag EY, El Badry Mohamed M, Mohamed GG, Ebrahim MS (2020) Selective potentiometric sensors for the determination of butenafine hydrochloride in a cream formulation. Microchem J 157:104870
Ghalwa NA, Almonem KIA, Al-Kashef ID, Saadeh SM, Shawish HMA (2020) Comparative effects of surfactants on the behavior of an anticancer drug potentiometric sensor. Anal Methods 12:679–686
Ghoniem MG, Mohamed MA, Eldin GMG, Errachid A (2021) Sensitive electrochemical strategy via the construction of functionalized carbon nanotubes/ionic liquid nanocomposite for the determination of anaesthetic drug cinchocaine. Measurement 185:110071
Herkenne C, Alberti I, Naik A, Kalia YN, Mathy F-X, Préat V, Guy RH (2008) In vivo methods for the assessment of topical drug bioavailability. Pharm Res 25:87
Hong-xiao C, Wei-bing L, Hua Y, Xi-ang Y (2010) Treatment of pityriasis versicolor with itraconazole in combination with butenafine cream and ketoconazole lotion:a clinical study, Chinese. Journal of Mycology 5:238–240
Kokjohn K, Bradley M, Griffiths B, Ghannoum M (2003) Evaluation of in vitro activity of ciclopirox olamine, butenafine HCl and econazole nitrate against dermatophytes, yeasts and bacteria. Int J Dermatol 42:11–17
Laviron E, Roullier L, Degrand C (1980) A multilayer model for the study of space distributed redox modified electrodes: part II theory and application of linear potential sweep voltammetry for a simple reaction. J Electroanal Chem Interfacial Electrochem 112:11–23
Mahdi WA, Bukhari SI, Imam SS, Alshehri S, Zafar A, Yasir M (2021) Formulation and optimization of butenafine-loaded topical nano lipid carrier-based gel: characterization irritation study, and anti-fungal activity. Pharmaceutics 13:1087
Miron D, Lange A, Zimmer AR, Mayorga P, Schapoval EE (2014) HPLC-DAD for the determination of three different classes of antifungals: method characterization, statistical approach, and application to a permeation study. Biomed Chromatogr 28:1728–1737
Mohamed MA (2018) Wearable miniaturized electrochemical sensors: benefits and challenges. Electrochemistry 15:147–185
Mohamed MA, Abdelwahab NS, Banks CE (2016) Electroanalytical sensing of the antimicrobial drug linezolid utilising an electrochemical sensing platform based upon a multiwalled carbon nanotubes/bromocresol green modified carbon paste electrode. Anal Methods 8:4345–4353
Mohamed MA, Atty SA, Merey HA, Fattah TA, Foster CW, Banks CE (2017a) Titanium nanoparticles (TiO2)/graphene oxide nanosheets (GO): an electrochemical sensing platform for the sensitive and simultaneous determination of benzocaine in the presence of antipyrine. Analyst 142:3674–3679
Mohamed MA, Atty SA, Merey HA, Fattah TA, Foster CW, Banks CE (2017b) Titanium nanoparticles (TiO 2)/graphene oxide nanosheets (GO): an electrochemical sensing platform for the sensitive and simultaneous determination of benzocaine in the presence of antipyrine. Analyst 142:3674–3679
Mohamed MA, Fayed AS, Hegazy MA, Salama NN, Abbas EE (2019) Fully optimized new sensitive electrochemical sensing platform for the selective determination of antiepileptic drug ezogabine. Microchem J 144:130–138
Mohamed MA, Salama NN, Sultan MA, Manie HF, El-Alamin MMA (2020) Cobalt ferrite magnetic nanoparticles as a highly efficient electrochemical platform for simultaneous determination of dexlansoprazole and granisetron hydrochloride. Microchem J 159:105424
Mohamed MA, Eldin GMG, Ismail SM, Zine N, Elaissari A, Jaffrezic-Renault N, Errachid A (2021) Innovative electrochemical sensor for the precise determination of the new antiviral COVID-19 treatment Favipiravir in the presence of coadministered drugs. J Electroanal Chem 895:115422
Mohd Norsham IN, Baharin SNA, Raoov M, Shahabuddin S, Jakmunee J, Sambasevam KP (2020) Optimization of waste quail eggshells as biocomposites for polyaniline in ammonia gas detection. Polym Eng Sci 60:3170–3182
Nahm WK, Orengo I, Rosen T (1999) The antifungal agent butenafine manifests anti-inflammatory activity in vivo. J Am Acad Dermatol 41:203–206
Parfitt NR, Skinner MF, Bon C, Kanfer I (2011) Bioequivalence of topical clotrimazole formulations: an improved tape stripping method. J Pharm Pharm Sci 14:347–357
Saadeh S, Tamous H, Tbaza A (2018) Determination of atomoxetine hydrochloride in biological fluids using potentiometric carbon paste electrode modified by TiO2 nanoparticles. Acta Chim Slov 65:811–822
Sakagami Y (2010) Antifungal drugs mode of action and recent development. In: Combating fungal infections, Springer, pp 99–107
Sanghavi BJ, Srivastava AK (2013) Adsorptive stripping voltammetric determination of imipramine, trimipramine and desipramine employing titanium dioxide nanoparticles and an Amberlite XAD-2 modified glassy carbon paste electrode. Analyst 138:1395–1404
Song L, Jiang X, Wang L (2011) Determination of butenafine hydrochloride in human plasma by liquid chromatography electrospray ionization-mass spectrometry following its topical administration in human subjects. J Chromatogr B 879:3658–3662
Syed TA, Maibach HI (2000) Butenafine hydrochloride: for the treatment of interdigital tinea pedis. Expert Opin Pharmacother 1:467–473
Teichmann A, Heuschkel S, Jacobi U, Presse G, Neubert RH, Sterry W, Lademann J (2007) Comparison of stratum corneum penetration and localization of a lipophilic model drug applied in an o/w microemulsion and an amphiphilic cream. Eur J Pharm Biopharm 67:699–706
Yarin AL, Pourdeyhimi B, Ramakrishna S (2014) Fundamentals and applications of micro-and nanofibers, Cambridge University Press
Zhang W, He Z, Han Y, Jiang Q, Zhan C, Zhang K, Li Z, Zhang R (2020) Structural design and environmental applications of electrospun nanofibers. Compos Part A Appl Sci Manuf 137:106009
Funding
Open access funding provided by The Science, Technology & Innovation Funding Authority (STDF) in cooperation with The Egyptian Knowledge Bank (EKB).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Mohamed, M.A., Salama, N.N., Sultan, M.A. et al. Sensitive and effective electrochemical determination of butenafine in the presence of itraconazole using titanium nanoparticles-ionic liquid based nanocomposite sensor. Chem. Pap. 77, 1929–1939 (2023). https://doi.org/10.1007/s11696-022-02593-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11696-022-02593-3