Abstract
Nanotechnology has a real-world impact on every aspect of life. Many researchers have been drawn to the biosynthesis of gold and iron oxide nanoparticles (Au-NPs and SPIONS) because they have a wide range of life applications. In this work, a single-step environmentally friendly biosynthesis of Au-NPs and SPIONS is reported by reducing solutions of gold aureate and ferric chloride is reported for the first time using the hydroethanolic extract (HEE) of Salvia officinalis (S. officinalis), an edible plant found in Egypt. The phytochemicals present in HEE were responsible for the reduction as well as stabilization of these nanoparticles. Before using the HEE, it was phytochemically screened for its constituents. Qualitatively, the HEE was found to have comparable levels of phenolics, flavonoids, tannins, proteins, carbohydrates, terpenoids, steroids, and polysaccharides. Quantitatively, total phenolics (236.91 ± 2.15 mg GAE/g extract), flavonoids (91.38 ± 0.97 mg QE/g extract), tannins (101.60 ± 1.33 mg/g extract), proteins (284.62 ± 2.65 mg/g extract), carbohydrates (127.73 ± 1.68 mg/g extract), soluble sugars (52.3 ± 0.67 mg/g extract), and polysaccharides (75.43 ± 1.01 mg/g extract) were estimated. In addition, HPLC analysis revealed the identification of seven phenolic compounds [ferulic (67.26%), chlorogenic (3.12%), caffeic (3.11%), p-coumaric (1.13%), protocatechuic (0.65%), catechin (0.69%), rosmarinic (0.53%)] and three flavonoids [apigenin (5.29%), quercetin-7-O-glucoside (3.39%), and luteolin-7-O-rutinose (2.01%)]. The characterization of the biosynthesized NPs was confirmed by Fourier transform infrared (FT-IR) spectroscopy, UV–Vis absorption spectroscopy, X-ray diffraction (XRD), and transmission electron microscopy (TEM). In vitro cytotoxic studies showed that Au-NPs, SPIONS, and HEE have an inhibitory effect on the growth of human breast cancer (MCF-7) cells at an IC50 of 6.53, 6.97, and 26.12 µg mL−1, respectively, by comparison with the standard drug (Doxorubicin) effect (0.18 µg mL−1).
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Biosynthesis is one of the most crucial routes for environmentally friendly synthesizing NPs via various biological materials that act as reducing and capping agents for the synthesized NPs. Microorganisms, marine organisms, and plant extracts are among them (Bao and Lan 2019; John Sushma et al. 2016; Priyanka et al. 2016; Premanand et al. 2016; Jafar et al. 2015; Medda et al. 2015; El-Rafie et al. 2013). However, microorganisms require controlled conditions like pH, temperature, and maintenance of culture media and other factors for growth. Plant extracts, on the other hand, are more economical, widely available, safe, and environmentally friendly than other biological methods for the biosynthesis of NPs (Pirtarighat et al. 2017; Ganeshkumar et al. 2013; Kharissova et al. 2013; Cai et al. 2010). Extracts of various plant parts contain natural metabolites such as steroids, saponins, alkaloids, flavonoids, tannins, and other nutritional compounds, retaining the unique properties of NPs as well as the therapeutic potency of the plant extract.
Salvia, the largest genus in the Lamiaceae family, is endemic to the Mediterranean region and the Middle East (Walker et al. 2004). Plants in this genus have a wide range of traditional medicinal uses (Ghorbani and Esmaeilizadeh 2017; Adams et al. 2007). Terpenes, alkaloids, fatty acids, carbohydrates, glycosidic derivatives, phenolic compounds, polyacetylenes, steroids, and diterpenoids are the primary phytoconstituents in S. officinalis leaves, stems, and flowers (Ghalem and Ali 2017; Kadhim et al. 2016; Kontogianni et al. 2013). So, extracts from different parts of S. officinalis may serve as efficient biosynthesizers and stabilizers for a variety of metal and metal oxide nanoparticles.
Cancer is now the second leading cause of death, trailing cardiovascular disease. Detection and treatment of cancer in its early stages remains a major challenge, despite rapid advancements in surgery, diagnostic techniques, chemotherapy, and pharmaceutical chemistry. Breast cancer is the leading cause of death in women (Tanih and Ndip 2013). Despite being the least common type of cancer, bioactive metabolites from Salvia species have been reported to have cytotoxic activity, particularly on breast cancer cell lines (Yıldırım and Kutlu, 2015; Kafil et al. 2015; Alzeer et al. 2014; Loizzo et al. 2014; Özer et al. 2013).
In addition, the biosynthesis of Au-NPs and SPIONS was both environmentally and economically friendly. Both have numerous advantages because of their applicable surface chemistry, which exhibits many interesting properties that can be used in a variety of biomedical applications such as tissue repair, drug delivery, hyperthermia, and cell separation (Vita et al. 2018; Siddiqi et al. 2016). They also helped to solve some of the problems that come with current breast cancer treatment methods.
Based on the aforementioned facts, the objectives of this study were to synthesize and characterize Au-NPs and SPIONS, for the first time, using S. officinalis HEE after its phytochemical investigation. Their in vitro cytotoxicity was assessed against human breast cancer cell lines (MCF-7).
Experimental
Materials
Iron(III) chloride (FeCl3, 99.9%; MW = 162.2) and Tetrachloroauric(III) acid trihydrate (HAuCl4. 3H2O, 99.9%; MW = 393.83) were supplied by Sigma-Aldrich, USA, and used as iron oxide and gold nanoparticle precursors, respectively. Other chemicals were of analytic quality and were used as received, with no further purification. All aqueous solutions were made with double-distilled water. Before use, all glassware was thoroughly cleaned and washed with distilled water before being dried.
Plant extraction, preparation, and characterization
Leaves of S. officinalis were obtained from an honest spice dealer in Cairo, Egypt. The dried, powdered plant of S. officinalis (100 g) was extracted with 70% ethanol using a Soxhlet apparatus until the extraction was complete, and then the ethanol was filtered out. The solvent was removed under reduced pressure at about 45 °C. The residue (2.54%) of the hydroethanolic extract (HEE) was stored until further laboratory analysis.
Different plant phytochemicals, including terpenoids/steroids, tannins, carbohydrates (reducing sugars), flavonoids, phenolic compounds, saponins, cardiac glycosides, alkaloids, proteins/amino acids, and anthraquinones, were all screened qualitatively according to previously reported methods (AOAC 2016; Shinde et al. 2012; Prabhu et al. 2011; Parekh and Chanda 2007).
The quantitative determination of the aforesaid constituents, including total flavonoids, phenolics, tannins, proteins, total carbohydrates, and soluble sugars, was achieved according to the following procedures:
Total flavonoids
A colorimetric method was used to calculate the total flavonoid content (Haq et al. 2019). In a test tube, 4 mL of distilled water (DH2O) was added to 1 mL of the HEE, followed by the addition of 0.3 mL of sodium nitrite solution (5%) and 0.3 mL of aluminium chloride solution (10%). The test tube was incubated for five min, and then 2 mL of sodium hydroxide (1 M) was added to the reaction mixture. The reaction volume was increased to 10 mL with DH2O. The test tube was placed in the shaker and the absorption of the colour developed was measured at 510 nm. For the calibration, an aqueous solution of known quercetin concentrations was used, and the results were given in milligrams of quercetin equivalents (QE) per gram of extract.
Total phenolic content (TPC)
The colorimetric technique was used to determine the TPC of the examined plant using the Folin–Ciocalteu method with some modification (Haq et al. 2019). According to this technique, 0.5 mL of the extract solution was mixed in a test tube with 0.25 mL of Folin–Ciocalteu and 2.25 mL of methanol and stirred for 1 min before being placed in the dark for 8 min. After that, the mixture was incubated for 120 min at 25 °C with 2.0 mL of sodium carbonate (7.5% w/v). The absorption was measured at 756 nm, relative to the blank (methanol). Concentrations of total phenolic compounds were determined as milligrams of gallic acid equivalent/g extract (mg GAE)/g HEE) using the regression equation from the calibration curve of the gallic acid standard. The experiment was conducted in triplicate.
Total tannin content
The tannins were determined using the Folin-Ciocalteu method with some modifications (Kavitha Chandran and Indira 2016). About 0.1 mL of the plant extract was added to a volumetric flask (10 mL) containing 7.5 mL of distilled water, 0.5 mL of Folin-Ciocalteu phenol reagent, and 1 mL of 35% sodium carbonate solution, and was then diluted to 10 mL with distilled water. The mixture was shaken well and kept at room temperature for 30 min. A set of reference standard solutions of tannic acid was prepared. Absorbance for the test and standard solutions was measured against the blank (distilled water) at 700 nm. The estimation of the total tannin content was carried out in triplicate.
Protein content
The protein content, expressed as the total nitrogen content, was calculated using the micro-Kjeldahl apparatus by the Pearson procedure (Pearson 1970).
Total sugars
The anthrone technique, defined by Umbriet et al. (1959), was used to calculate total sugars. Six mL of anthrone solution (2 g/L of H2SO4, 95%) were added to a 3 mL sample and placed in a boiling water bath for 3 min. After cooling, the established colour was spectrophotometrically measured at 620 nm.
HPLC conditions of phenolics and flavonoids
A quantitative identification of the HEE phenolic constituents was performed using HPLC analysis according to the method described in detail by El-Rafie et al. (2016). The HPLC analysis was conducted with the Agilent 1260 series. Chromatographic separation was achieved using the Eclipse C-18 4.6 mm × 250 mm column (5 μm particle size). At a flow rate of 1 mL/min, the mobile phase was made up of water (A) and trifluoroacetic acid (0.05%) in acetonitrile (B). The mobile phase was sequentially programmed in a linear gradient as follows: 0 min (82% A); 0–5 min (80% A); 5–8 min (60% A); 8–12 min (6% A); 12–15 min (85% A); and 15–16 min (82% A). At 280 nm, the multi-wavelength detector was monitored. The injection volume for each of the sample solutions was 10 μL. The column's temperature was kept constant at 35 °C.
Biosynthesis and characterization of nanoparticles
Gold nanoparticles (Au-NPs) synthesis
10 mL of HEE of S. officinalis were added to a certain amount of double-distilled water, and then 1 mL of 0.1 M chloroauric acid (HAuCl4) solution was added to this extract solution. An aqueous solution of NaOH (100 mmol/L) was added dropwise until the mixture's pH reached 13, and the total volume was then completed to 100 mL with distilled water. The mixture was continuously stirred with a magnetic stirrer with different time intervals (15, 30, 45, and 60 min) and at a temperature of 60 °C. The formation of Au-NPs is indicated by a colour change from pale yellow to reddish-violet.
Iron oxide nanoparticles (SPIONS) synthesis.
A known weight of ferric chloride (0.93 g) was dissolved in 10 mL of double distilled water for 15 min with continuous stirring. The resultant solution was mixed with 10 mL of the HEE from S. officinalis. The volume was then adjusted to 60 mL with distilled water, and the procedure was then performed as for Au-NPs synthesis. Within 10 min., the colour changes from yellow to deep green, indicating the formation of SPIONS.
Characterization of NPs
The following approaches (González-Ballesteros et al. 2017; Salem et al. 2019) were used to identify the produced nanoparticles:
-
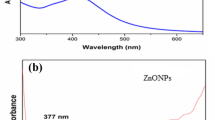
Visual and UV–Vis: SPIONS at 200–500 nm and Au-NPs at 450–600 nm were measured using a spectrophotometer (Jasco V-670 UV-V), as shown in Figs. 1a and b, respectively.
-
FT-IR analysis: Au-NPs and SPIONS were singly purified using repeated centrifugation at 8000 rpm for 16 min and redistributed into 10 mL of deionized water. After drying, the synthesized nanoparticles were subjected to FTIR analysis, and the FTIR spectra were performed and recorded with a Fourier transform infrared spectrophotometer of type Nicolet 870 between 4000 and 400 cm−1 with a resolution of 4 cm−1 as shown in Fig. 2a, b.
-
XRD analysis: The oven-dried nanoparticles were coated onto an XRD grid and analyzed by an X-ray diffractometer (6000-shimadzu-Japan) (Fig. 3a, b).
-
TEM analysis: TEM-2100 (JEOL Japan) was used to determine the particle size, distribution, and morphology of the formed nanoparticles (Fig. 4a, b).
In vitro cytotoxic activity
The anticancer activities of S. officinalis HEE, biosynthesized Au-NPs, and SPIONS against the MCF-7 breast cancer cell line (ATCC® HTB-22™) were investigated using an in vitro MTT assay. Cell lines obtained from the American Type Culture Collection (Manassas, VA, USA) were maintained in the recommended RPMI-1640 and D10 medium consisting of Dulbecco’s complete medium (Invitrogen, Carlsbad, CA) supplemented with 10% heat-inactivated (56 °C) fetal bovine serum, penicillin (100 IU/mL), l-glutamine (3 mM), and streptomycin (100 mg/mL). An incubator of 95% air and 5% CO2 at 37 °C with an atmosphere was used for cell growth. The MCF-7cell line was exposed to HEE, Au-NPs, SPIONS, and Doxorubicin (standard drug) in various concentrations; 100, 50, 25, 12.5, 6 μg/mL, and 0 concentrations for control wells (only the nutrient medium was added to the cells) for 48 h later. Cell survival was determined using the 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide (MTT) assay, according to Van Meerloo et al. (2011). Briefly, cells were allowed to adhere for one day in a CO2 incubator at 37 °C in 96-well culture plates. After the respective exposures, MTT (5 mg/mL of stock in PBS) was added and the plates were incubated further for 4 h. Then, supernatants were thrown in, each well mixed gently after the addition of 200 μL of DMSO. The intensity of the absorbance of light was proportional to the number of viable cells in each well, which was read in an enzyme-linked immunosorbent assay (ELISA) plate reader by Biotek (ELX-800) at 570 nm. All experiments were performed in triplicate and expressed as mean ± SD:
Results and discussion
Proximate analysis is a cost-effective and easy method for the determination of the nutritional value of plants. The phytochemical screening results shown in Table 1 show that the HEE of S. officinalis leaves contains a wide range of bioactive compounds, including carbohydrates (+ + +), flavonoids (+ +), phenolics (+ + +), terpenoids ( +), steroids ( +), tannins (+ +), proteins/amino acids (+ + +), and alkaloids ( +), which are responsible for the biosynthesis of Au-NPs and SPIONS (Kanagasubbulakshmi and Kadirvelu 2017; Lee et al. 2015; Makarov et al. 2014; Mondal 2011).
The quantitative analysis of HEE shown in Table 2 revealed that proteins are the most abundant constituent (284.62 ± 2.65 mg/g), followed by phenolics (236.91 ± 2.15 mg GAE/g), carbohydrates (127.73 ± 1.68 mg/g), and tannins (101.60 ± 1.33 mg/g). Flavonoids (91.38 ± 0.97 mg QE/g), polysaccharides (75.43 ± 1.01 mg/g), and soluble sugars (52.30 ± 0.68 mg/g) were found to be minor components.
Our findings are consistent with previous research on Salvia species in which HEE (70%) contains more total phenolics than total flavonoid content (Mocan et al. 2020; Veliˇckovi´c et al. 2011). Concerning the phenolic profiles of Salvia species that have been studied before (Dib et al. 2021; Mocan et al. 2020; Afonso et al. 2019), our results agreed with the types of phenolics found but not with the amounts found.
The most widely used technique for estimating phenolic and flavonoid compounds in plants and seaweeds is high-performance liquid chromatography (HPLC) (Aguilar-Hernández 2017). The phenolic contents in the HEE of S. officinalis leaves were then accurately quantified and identified using HPLC analysis. Table 3 depicts the identification of six polyphenol carboxylic acids, which represent a large group of naturally occurring organic compounds having a wide range of medicinal activities, particularly cytotoxic and antioxidant activities. These phenolic acids (mg/g extract) are either cinnamic acid derivatives, the most abundant of which is ferulic (67.26), followed by chlorogenic (3.12), caffeic (3.11), p-coumaric (1.13), and rosmarinic (0.53), or a benzoic acid derivative, protocatechuic (0.65). In addition, one flavan-3-ol, catechin, and one aglycon from the flavone class (apigenin 5.29) were identified, as well as two glycosides, quercetin-7-o-glucoside (3.39) and luteolin-7-o-rutinose (2.01). These compounds were found to possess potent antioxidant and cytotoxic activities (Umamaheswari et al. 2018; Zduńska et al. 2018; Liu et al. 2017; Maiyo et al. 2016; Shukla and Gupta 2010).
It is worth mentioning that most of the phenols and flavonoids identified in this study are similar to those found in the aqueous, methanolic, and ethanolic extracts of other Salvia species (Al-Jaber et al. 2020; Fotovvat et al. 2019; Hanganu et al. 2019; Grzegorczyk-Karolak and Kiss 2018).
Following characterization, the HEE of S. officinalis leaves was used separately for the biosynthesis of Au-NPs and SPIONS via the reduction of their respective salts, with the process taking just a few minutes to complete. The formation of NPs was initially evidenced by the change in colour of the solution from light-yellow to reddish-violet in the case of Au-NPs and deep green in the case of SPIONS. The intensity of the colour is deepened by time as nanoparticle formation is directly proportional to time. The reducing properties of the hydroxyl (OH) groups in HEE phytochemicals (Tables 1, 2, 3) may be responsible for the formation of these nanoparticles. The presence of these phyto-reductants in an aqueous alkaline solution with HAuCl4 or FeCl3 may result in the following redox reactions (Fig. 1):
The high alkalinity of the reaction medium neutralizes HCl once it is formed (Eqs. 3 and 5), shifting the reaction direction between the HEE phenolics and HAuCl4 or FeCl3 towards the formation of Au-NPs or SPIONS. This could explain why these nanoparticles only form in a highly alkaline solution (i.e., pH13). Also, active HEE phytochemicals like phenolics, flavonoids, terpenoids, polysaccharides, tannins, and proteins (Tables 1, 2) play an important role in the capping of the Au-NPs and SPIONS (stabilization and protection from aggregation) in the aqueous medium (Fig. 2), as has been reported before (Zayadi and Bakar 2020).
The formation of NPs was also confirmed by UV–visible surface measurements, as shown in Fig. 3, where the typical plasmon peaks for Au-NPs and SPIONS are in the ranges of 275–325 nm and 525–540 nm, respectively.
The HEE constituents involved in the reduction and capping of the nanoparticle can be identified by the FTIR technique. Figure 4 shows that the FTIR of the HEE, Au-NPs, and SPIONS has a broad peak at 3464 cm−1, which corresponds to the O–H stretch, which may be H-bonded to phenolics and alcohols. At 2921 cm−1, a narrow band was seen, suggesting the existence of C-H stretched alkane groups. Broadbands were also observed at 1629 cm−1, which corresponds to similar conjugation effects of N–H stretching; 1372 cm−1 corresponds to the N–O stretch of nitro compounds; 1109 cm−1 is characteristic of the N–H stretch of aliphatic or aromatic amines, and a narrow band at 667 cm−1 is due to the bending vibrations of N–H groups in proteins. There have been other studies that say the wavenumber signal of the previous peaks may be caused by phytoconstituents found in plant extracts like proteins, alkaloids, terpenoids, flavonoids, and phenolics. These phytoconstituents may be responsible for reducing and stabilizing the biosynthesized nanoparticles (Singh et al. 2018; El-Rafie et al. 2017; Sengani 2017; Kalpana et al. 2016).
The XRD spectrum identified the crystalline structure of the biosynthesized NPs of gold and iron oxides (Fig. 5). The reduced NPs have different diffraction peaks at 2 Theta values of 38.12°, 44.25°, 64.7°, 77.5°, and 81.8° in the case of Au-NPs and 41°, 66°, and 85° in the case of SPIONS, which correspond to the crystal planes of (111), (200), (220), (311), and (222) of crystalline Au-NPs (JCPDS file No. 02–1095) (Van-Dat et al. 2020) and (110), (200), and (211) of crystalline SPIONS (JCPDS file No. 01–1252) (Parimala and Santhanalakshmi, 2014), respectively. This result confirmed the nanoparticle crystalline structure. The particle size is obtained by taking the average of the sizes at the peaks using the Debye–Scherrer equation (Naganthran et al., 2022), as shown in Tables 4 and 5 for Au-NPs and SPIONS, respectively. They were found to be 9.27 nm and 64.95 nm, respectively.
Where D is the crystal size, λ is the wavelength of X-ray, ẞ is the full-width at half-maximum (FWHM) value of XRD diffraction lines, Ɵ is the half-diffraction value of 2Ɵ and K = 0.9 is a constant.
The biosynthesized Au-NPs and SPIONS were further studied using transmission electron microscopy (TEM). The latter has an average core dimension of 20–25 nm for Au-NPs and 30–35 nm for SPIONS, according to the TEM images and particle size distribution histograms in Fig. 6. As shown in these photos, the particles are about spherical and hexagonal in shape (Fig. 6).
Because Au-NPs are inert, they are non-toxic and safe to use; thus, their biomedical applications, particularly in cancer treatment and diagnostic tools, have been extensively researched (Anik et al. 2021; Nashat and Haider 2021; Sim and Wong 2021). The MTT assay was used to compare the cytotoxic activity of biosynthesized Au-NPs and SPIONS against the human breast cancer (MCF-7) cell line and its related HEE and Doxorubicin as a standard drug at different concentrations (6.25, 12.5, 25, 50, and 100 l/mL). Table 6 and Fig. 7 show the results. Both nanoparticles and their related HEE produced dose-dependent cell inhibition; the inhibitory concentration (IC50 value) for Au-NPs, SPIONS, and HEE, respectively, was 6.53, 6.97, and 26.12 µg mL−1.
Because of their thermal ablation, photodynamic property, and angiogenesis, Au-NPs are cytotoxic to cancer cells. On the other hand, the ability of SPIONS to kill tumor cells may be explained by the production of reactive oxygen species or hyperthermia (Aswathanaryan 2018; Silva et al. 2011; Johannsen et al. 2010). It is likely that HEE has cytotoxic effects because it contains phenolics, especially rosmarinic acid, which has been shown to stop the growth of many human cancers (Yesil-Celiktas et al. 2010; Xavier et al. 2009).
The surface area to volume ratio of Au-NPs and SPIONS nanoparticles is higher than that of HEE. This allows more atoms to be on the surface, as well as easier cell membrane penetration and interaction with intracellular materials, which leads to easier cell destruction.
Conclusions
The current work reveals that Au-NPs and SPIONS can be synthesized in an environmentally friendly manner by using the hydroethanolic extract (HEE) of S. officinalis leaves, where the phytochemicals in this extract serve as both reducing and capping agents for the formed nanoparticles. This simple and easy method has various benefits, which include biocompatibility, cost-effectiveness, and ease of scale-up production. The profitable biosynthesis of both Au-NPs and SPIONS was proved by different techniques such as FTIR, UV–visible spectroscopy, TEM, and XRD. The results of this study reveal that both Au-NPs and SPIONS have potent in vitro cytotoxic effects on MCF-7 (a human breast cancer cell line). Future research would include the validation of environmentally friendly manufactured Au-NPs and SPIONS that could be used in cancer therapy. Accordingly to the results of this work, S. officinalis HEE can be used efficiently to synthesize green Au-NPs as well as SPIONS, which can be used as anticancer agents. The NPs generated in this way could also be used in the biomedical field to discover potential therapeutic agents for a range of human diseases.
References
Adams M, Gmünder F, Hamburger M (2007) Plants traditionally used in age related brain disorders—a survey of ethnobotanical literature. J Ethnopharmacol 113:363–381. https://doi.org/10.1016/j.jep.2007.07.016
Afonso AF, Pereira OR, Fernandes Â, Calhelha RC, Silva AMS, Ferreira ICFR, Cardoso SM (2019) Phytochemical composition and bioactive effects of Salvia africana, Salvia officinalis ‘Icterina’ and Salvia mexicana aqueous extracts. Molecules 24(23):4327. https://doi.org/10.3390/molecules24234327
Aguilar-Hernández I, Afseth NK, López-Luke T, Contreras-Torres F, Wold JP, Ornelas-Soto N (2017) Surface enhanced Raman spectroscopy of phenolic antioxidants: a systematic evaluation of ferulic acid, p-coumaric acid, caffeic acid and sinapic acid. Vib Spectrosc 89: 113–122. Doi: https://doi.org/10.1016/j.vibspec .2017.02.002.
Al-Jaber HI, Shakya AK, Elagbar ZA (2020) HPLC profiling of selected phenolic acids and flavonoids in Salvia eigii, Salvia hierosolymitana and Salvia viridis growing wild in Jordan and their in vitro antioxidant activity. PeerJ 8:e9769. https://doi.org/10.7717/peerj.9769
Alzeer J, Vumiddi BR, Arefeh R, Rimawi W, Saleem H, Luedtke NW (2014) The influence of extraction solvents on the anticancer activities of Palestinian medicinal plants. Res J Med Plant 8: 408–415
Anik MI, Mahmud N, Al Masud A, Hasan M (2021) Gold nanoparticles (GNPs) in biomedical and clinical applications: areview. Nano Select 2021:1–37. https://doi.org/10.1002/nano.202100255
AOAC International (2016) Official methods of analysis, 20th edition. AOAC International, Rockville, Maryland, USA, pp 2085–3250.
Aswathanarayan JB, Vittal RR, Muddegowda U (2018) Anticancer activity of metal nanoparticles and their peptide conjugates against human colon adenorectal carcinoma cells. Artif Cells Nanomed Biotechnol 46:1444–1451. https://doi.org/10.1080/21691401.2017.1373655
Bao Z, Lan CQ (2019) Advances in biosynthesis of noble metal nanoparticles mediated by photosynthetic organisms-A review. Colloids Surf B Biointerfaces 184:110519. https://doi.org/10.1016/j.colsurfb.2019.110519
Cai Y, Shen Y, Xie A, Li S, Wang X (2010) Green synthesis of soya bean sprouts-mediated superparamagnetic Fe3O4 nanoparticles. J Magn Magn Mater 322: 2938−2943. Doi: https://doi.org/10.1016/j.jmmm.2010.05.009.
Dib H, Seladji M, Bencheikh FZ, Faradji M, Benammar C, Belarbi M (2021) Phytochemical screening and antioxidant activity of Salvia hispanica. J Pharm Res Int 33(41A): 167–174. Doi: https://doi.org/10.9734/jpri/2021/v33i41A32314
El-Rafie HM, Abd El-Aziz SM, Zahran MK (2016) Bioactivities of gold and iron oxide nanoparticles biosynthesized from the edible plant Corchorus olitorius. Pharm Lett 8:156–164
El-Rafie HM, El-Rafie MH, Zahran MK (2013) Green synthesis of silver nanoparticles using polysaccharides extracted from marine macro algae. Carbohydr Polym 96: 403–410. Doi: https://doi.org/10.1016/j.carbpol.2013.03.071
El-Rafie HM, El-Rafie MH, Zahran MK (2017) Anti-inflammatory and antibacterial activities of nanosilver treated cotton fabric prepared from ethanolic extracts of three Terminalia species. Egypt J Chem 60: 129–142. DOI: https://doi.org/10.21608/EJCHEM.2017.1607.1132
Fotovvat M, Radjabian T, Saboora A (2019) HPLC fingerprint of important phenolic compounds in some Salvia L. species from Iran. Rec Nat Prod 13:37–49. Doi: https://doi.org/10.25135/rnp.72.18.02.228.
Ganeshkumar M, Sathishkumar M, Ponrasu T, Dinesh MG, Suguna L (2013) Spontaneous ultra-fast synthesis of gold nanoparticles using punica granatum for cancer targeted drug delivery. Colloids Surf B Biointerfaces 106:208–216. https://doi.org/10.1016/j.colsurfb.2013.01.035
Ghalem BR, Ali B (2017) Preliminary phytochemical screening of five commercial essential oils. World J Appl Chem 2: 145–151. Doi: https://doi.org/10.11648/j.wjac.20170204.16.
Ghorbani A, Esmaeilizadeh M (2017) Pharmacological properties of Salvia officinalis and its components. J Tradit Complement Med 7:433–440
González-Ballesteros N, Prado-López S, Rodríguez-González JB, Lastra M, Rodríguez-Argüelles MC (2017) Green synthesis of gold nanoparticles using brown algae Cystoseira baccata: Its activity in colon cancer cells. Colloids Surf B Biointerfaces 153:190–198. https://doi.org/10.1016/j.colsurfb.2017.02.020
Grzegorczyk-Karolak I, Kiss AK (2018) Determination of the phenolic profile and antioxidant properties of Salvia viridis L. Shoots: a comparison of aqueous and hydroethanolic extracts. Molecules 23:1468. Doi: https://doi.org/10.3390/molecules23061468
Hanganu D, Olah NK, Pop CE, Vlase L, Oniga I, Ciocarlan N, Matei A, Pus cas, Silaghi-Dumitrescu R, Benedec D (2019) Evaluation of polyphenolic profile and antioxidant activity for some Salvia species. Farmacia 67: 801–805. Doi: https://doi.org/10.31925/farmacia.2019.5.8.
Haq SH, Al-Ruwaished G, Al-Mutlaq MA, Naji SA, Al-Mogren M, Al-Rashed S, Tul Ain Q, Al-Amro A A, Al-Mussallam A (2019) Antioxidant, anticancer activity and phytochemical analysis of green algae, Chaetomorpha collected from the Arabian Gulf. Sci Rep 9::18906. Doi: https://doi.org/10.1038/s41598-019-55309-1.
Jafar A, Shama Z, Naeem A (2015) Green synthesis of metal nanoparticles by microorganisms; a current prospective. J Nanoanalysis 2:32–38. https://doi.org/10.1186/s12951-021-00834-3
Johannsen M, Thiesen B, Wust P, Jordan A (2010) Magnetic nanoparticle hyperthermia for prostate cancer. Int J Hyperthermia 26:790–795. https://doi.org/10.3109/02656731003745740
John Sushma N, Prathyusha D, Swathi G, Madhavi T, Deva Prasad Raju B, Mallikarjuna Kim HS (2016) Facile approach to synthesize magnesium oxide nanoparticles by using Clitoria ternatea: characterization and in-vitro antioxidant studies. Appl Nanosci 6: 437–444. Doi: https://doi.org/10.1007/s13204-015-0455-1.
Kadhim SM, Mohammed MT, Ahmed OM, Noori Jassim AM (2016) Study of some Salvia officinalis L. (sage) components and effect of their aqueous extract on antioxidant. Int J Chem Sci 14:711–719
Kafil V, Eskandandi M, Omidi Y, Nazemiyeh H, Barar J (2015) Abietane diterpenoid of Salvia sahendica Boiss and Buhse potently inhibits MCF-7 breast carcinoma cells by suppression of the PI3K/AKT pathway. RSC Adv 5:18041–18050. https://doi.org/10.1039/c4ra14905j
Kalpana VN, Chakraborthy P, Palanichamy V, Rajeswari VD (2016) Synthesis and characterization of copper nanoparticles using Tridax procumbens and its application in degradation of Bismarck brown. Int J Chemtech Res 9:498–507
Kavitha Chandran CI, Indira G (2016) Quantitative estimation of total phenolic, flavonoids, tannin and chlorophyll content of leaves of Strobilanthes Kunthiana (Neelakurinji). J Med Plants Stud 4:282–286
Kanagasubbulakshmi S, Kadirvelu K (2017) Green synthesis of iron oxide nanoparticles using Lagenaria Siceraria and evaluation of its antimicrobial activity. Def Life Sci J 2: 422–427. Doi: 10.14429/ DLSJ.2.12277
Kharissova OV, Dias HV, Kharisov BI, Perez BO, Perez VM (2013) The greener synthesis of nanoparticles. Trends Biotechnol 31: 240−248. Doi: https://doi.org/10.1016/j.tibtech.2013.01.003
Kontogianni VG, Tomic G, Nikolic I, Nerantzaki AA, Sayyad N, Stosic-Grujicic S, Stojanovic I, Gerothanassis IP, Tzakos AG (2013) Phytochemical profile of Rosmarinus officinalis and Salvia officinalis extracts and correlation to their antioxidant and anti-proliferative activity. Food Chem 136:120–129. https://doi.org/10.1016/j.foodchem.2012.07.091
Lee KD, Nagajyothi PC, Sreekanth TVM, Park S (2015) Eco-friendly synthesis of gold nanoparticles (AuNPs) using Inonotus obliquus and their antibacterial, antioxidant and cytotoxic activities. J Ind Eng Chem 26:67–72. https://doi.org/10.1016/j.jiec.2014.11.016
Liu R, Ji P, Liu B, Qiao H, Wang X, Zhou L, Deng T, Yi B (2017) Apigenin enhances the cisplatin cytotoxic effect through p53 modulated apoptosis. Oncol Lett 13: 1024–1030. Doi: https://doi.org/10.3892/ol.2016.5495.
Loizzo MR, Abouali M, Salehi P, Sonboli A, Kanani M, Menichini F, Tundis R (2014) In vitro antioxidant and antiproliferative activities of nine Salvia species. Nat Prod Res 28: 2278–2285. Doi: https://doi.org/10.1080/14786419. 2014.939086
Maiyo FC, Moodley R, Singh M (2016) Cytotoxicity, antioxidant and apoptosis studies of quercetin-3-O glucoside and 4-(β-D-glucopyranosyl-1→4-α-L-amnopyranosyloxy)-benzylisothiocyanate from Moringa oleifera, Anticancer Agents. J Med Chem 16:648–656. https://doi.org/10.2174/1871520615666151002110424
Makarov VV, Makarova SS, Love AJ, Sinitsyna OV, Dudnik AO, Yaminsky IV, Taliansky ME, Kalinina NO (2014) Biosynthesis of stable iron oxide nanoparticles in aqueous extracts of Hordeum vulgare and Rumex acetosa plants. ACS Langmuir 30:5982–5988. https://doi.org/10.1021/la5011924
Medda S, Hajra A, Dey U, Bose P, Mondal NK (2015) Biosynthesis of silver nanoparticles from Aloe vera leaf extract and antifungal activity against Rhizopus sp. and Aspergillus sp. Appl Nanosci 5:875–880. https://doi.org/10.1007/s13204-014-0387-1
Mocan A, Babotă M, Pop A, Fizeșan I, Diuzheva A, Locatelli M, Carradori S, Campestre C, Menghini L, Sisea CR, Sokovic M, Zengin G, Păltinean R, Bădărău S, C. Vodnar D, Crișan G (2020) Chemical constituents and biologic activities of sage species: A comparison between Salvia officinalis L., S. glutinosa L. and S. transsylvanica (Schur ex Griseb. & Schenk) Schur. Antioxidants 9(6):480. Doi: https://doi.org/10.3390/antiox9060480
Mondal S, Roy NR, Laskar A, Sk I, Begum NA (2011) Biogenic synthesis of Ag, Au and bimetallic Au/Ag alloy nanoparticles using aqueous extract of mahogany (Swietenia mahoagoni JACQ.) leaves. Colloids Surf B Biointerfaces 82:497–504. https://doi.org/10.1016/j.colsurfb.2010.10.007
Naganthran A, Verasoundarapandian G, Khalid FE, Masarudin MJ, Zulkharnain A, Nawawi NM, Karim M, Che Abdullah CA, Ahmad SA (2022) Synthesis, characterization and biomedical application of silver nanoparticles. Materials 15: 427. Doi: https://doi.org/10.3390/ma15020427
Nashat N, Haider Z (2021) A Review on gold nanoparticles (GNPs) and their advancement in cancer therapy. Int J Nanomater Nanotechnol Nanomed 7: 19–25
Özer H, Altun A, Unver-Saraydın S, Soylu S, Göktaş S, Tuncer E, İnan DS, Köksal B, Kaya Temiz T, Tepe B, Şen M, Karaday K, Turan M (2013) Antitumoral effects of Salvia abscondifflora Greuter & Burdet Syn. Salvia Crytantha Montbret & aucher ex Benth. on breast cancer. Indian J Tradit Knowl 12:390–397
Parekh J, Chanda SV (2007) In vitro antimicrobial activity and phytochemical analysis of some Indian medicinal plants. Turk J Biol 31:53–58
Parimala L, Santhanalakshmi J (2014) Studies on the iron nanoparticles catalyzed reduction of substituted aromatic ketones to alcohols. J Nanoparticles 2014: Article ID 156868. Doi: https://doi.org/10.1155/2014/156868
Pearson D (1970) The chemical analysis of foods, 6th edn. J & A Churchill, London
Pirtarighat S, Ghannadnia M, Baghshahi S (2017) Antimicrobial effects of green synthesized silver nanoparticles using Melissa officinalis grown under in vitro condition. Nanomed J 4: 184–190. Doi: https://doi.org/10.22038/nmj.2017.03.007
Prabhu K, Karar PK, Hemalatha S (2011) A Comparative preliminary phytochemical screening on the leaves, stems and the roots of three Viburnum Linn. Species Der Pharmacia Sinica 2:81–93
Premanand G, Shanmugam N, Kannadasan N, Sathishkumar K, Viruthagiri G (2016) Nelumbo nucifera leaf extract mediated synthesis of silver nanoparticles and their antimicrobial properties against some human pathogens. Appl Nanosci 6:409–415. https://doi.org/10.1007/s13204-015-0442-6
Priyanka S, Yu-Jin K, Dabing Z, Deok-Chun Y (2016) Biological synthesis of nanoparticles from plants and microorganisms. Trends Biotechnol 34:588–599. https://doi.org/10.1016/j.tibtech.2016.02.006
Salem DM, Ismail MM, Aly-Eldeen MA (2019) Biogenic synthesis and antimicrobial potency of iron oxide (Fe3O4) nanoparticles using algae harvested from the Mediterranean Sea. Egypt Egypt J Aquat Res 45:197–204. https://doi.org/10.1016/j.ejar.2019.07.002
Sengani M (2017) Identification of potential antioxidant indices by green gold nanoparticles in hyperglycemic Wistar rats. Environ Toxicol Pharmacol 50:11–19. https://doi.org/10.1016/j.etap.2017.01.007
Shind PR, Patil PS, Bairagi VA (2012) Pharmacognostic, phytochemical properties and antibacterial activity of Callistemon viminalis leaves and stems. Int J Pharm Pharm Sci 4:406–408
Shukla S, Gupta S (2010) Apigenin: A promising molecule for cancer prevention. Pharm Res 27:962–978. https://doi.org/10.1007/s11095-010-0089-7
Siddiqi KS, Ur Rahman A, Tajuddin HA (2016) Biogenic fabrication of iron/iron oxide nanoparticles and their application. Nanoscale Res Lett 11:498. https://doi.org/10.1186/s11671-016-1714-0
Silva AC, Oliveira TR, Mamani JB, Malheiros SM, Malavolta L, Pavon LE, Sibov T Jr, Amaro TE, Tannús A, Vidoto EL, Martins MJ, Santos RS, Gamarra LF (2011) Application of hyperthermia induced by superparamagnetic iron oxide nanoparticles in glioma treatment. Int J Nanomedicine 6:591–603. https://doi.org/10.2147/IJN.S14737
Sim S, Wong NK (2021) Nanotechnology and its use in imaging and drug delivery (Review). Biomed Rep 14:42. https://doi.org/10.3892/br.2021.1418
Singh H, Du J, Singh P, Yi TH (2018) Ecofriendly synthesis of silver and gold nanoparticles by Euphrasia officinalis leaf extract and its biomedical applications. Artif Cells, Nanomed Biotechnol 46:1163–1170. https://doi.org/10.1080/21691401.2017.1362417
Tanih NF, Ndip RN (2013) The acetone extract of Sclerocarya birrea (Anacardiaceae) possesses antiproliferative and apoptotic potential against human breast cancer cell lines (MCF-7). Sci World J 7:1–7. https://doi.org/10.1155/2013/956206
Umamaheswari C, Lakshmanan A, Nagarajan N (2018) Green synthesis, characterization and catalytic degradation studies of gold nanoparticles against Congo red and methyl orange. J Photochem Photobio B: Biology 178:33–39. https://doi.org/10.1016/j.jphotobiol.2017.10.017
Umbriet WW, Burris RH, Stauffer JF, Cohen PP, Johnse WJ, Lee Page GA, Patter VR, Schneider WC (1959) Manometric Techniques a manual describing methods applicable to the study of tissue metabolism. Burgess Publishing Company, p 239
Van Meerloo J, Kaspers GJL, Cloos J (2011) Cell sensitivity assays: The MTT assay. Methods Mol Biol 731:237–245. https://doi.org/10.1385/1-59259-687-8:25
Van-Dat D, Anh TT, Thanh-Danh N, Van-Cuong N, Xuan-Thang C, Thi Lan-Huong N, Le VT (2020) Biosynthesis of gold nanoparticles using Litsea cubeba fruit extract for catalytic reduction of 4-nitrophenol. J Nanomater 2020:1–10. https://doi.org/10.1155/2020/4548790
Veliˇckovi´c DT, Karabegovi´c IT, Stojiˇcevi´c SS, Lazi´c ML, Marinkovi´c VD, Veljkovi´c VB, (2011) Comparison of antioxidant and antimicrobial activities of extracts obtained from Salvia glutinosa L. and Salvia officinalis L. Hem Ind 65:599–605. https://doi.org/10.2298/HEMIND110412034V
Vita F, Innocenti C, Secchi A, Albertini F, Grillo V, Fiore A, Cozzoli PD, Fernández CJ (2018) Colloidal Au/iron oxide nanocrystal heterostructures: Magnetic, plasmonic and magnetic hyperthermia properties. J Mat Chem C 6: 12329–12340. Doi: 1039/c8tc01788c
Walker JB, Sytsma KJ, Wink JM (2004) Salvia (Lamiaceae) is not monophyletic: implications for the systematics, radiation, and ecological specializations of Salvia and tribe Mentheae. Am J Bot 91:1115–1125. https://doi.org/10.3732/ajb.91.7.1115
Xavier CP, Lima CF, Fernandes-Ferreira M, Pereira-Wilson C (2009) Salvia fruticosa, Salvia officinalis, and rosmarinic acid induce apoptosis and inhibit proliferation of human colorectal cell lines: The role in MAPK/ERK pathway. Nutr Cancer 61:564–571. https://doi.org/10.1080/01635580802710733
Yesil-Celiktas O, Sevimli C, Bedir E, Vardar-Sukan F (2010) Inhibitory effects of rosemary extracts, carnosic acid and rosmarinic acid on the growth of various human cancer cell lines. Plant Foods for Hum Nutr 65:158–163. https://doi.org/10.1007/s11130-010-0166-4
Yıldırım I, Kutlu T (2015) Anticancer properties of different species of Salvia. Hacettepe J Biol & Chem 43: 91–97. Doi: https://doi.org/10.15671/HJBC.20154312548
Zayadi RA, Abu Bakar F (2020) Comparative Study on Stability, Antioxidant and Catalytic Activities of Bio-stabilized Colloidal Gold Nanoparticles using Microalgae and Cyanobacteria J. Environ. Chem. Eng. 8(4):103843. Doi: https://doi.org/10.1016/j.jece.2020.103843
Zduńska K, Dana A, Kolodziejczak A, Rotsztejn H (2018) Antioxidant properties of ferulic acid and its possible application. Skin Pharmacol Physiol 31:332–336. https://doi.org/10.1159/000491755
Acknowledgements
The authors would like to thank the Department of Chemistry, Faculty of Science, Helwan University, and the Pharmacognosy Department, National Research Centre, Cairo, Egypt, for their support.
Funding
Open access funding provided by The Science, Technology & Innovation Funding Authority (STDF) in cooperation with The Egyptian Knowledge Bank (EKB). The authors received no financial support for the research, authorship, and/or publication of this article.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
El-Rafie, H.M., El-Aziz, S.M.A. & Zahran, M.K. In vitro cytotoxicity against breast cancer using biogenically synthesized gold and iron oxide nanoparticles derived from the hydroethanolic extract of Salvia officinalis L. Chem. Pap. 77, 361–373 (2023). https://doi.org/10.1007/s11696-022-02464-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11696-022-02464-x