Abstract
Heavy metal ions are among the most dangerous contaminants, which can cause serious health problems. In this work, ion-exchange resin beads were used as supports for magnetite (Fe3O4) synthesis to produce heavy metal adsorbents which can be easily separated by magnetic field. The first step of the magnetite preparation was the replacement of hydrogen ions with Fe2+ and Fe3+ ions on the sulfonic acid groups of the resin. In the second step, magnetite particle formation was induced by coprecipitating the iron ions with sodium hydroxide. The regeneration of the ion-exchange resin was also carried out by using sodium hydroxide. SEM images verified that relatively large magnetite crystal particles (diameter = 100–150 nm) were created. The ion-exchange effect of the prepared magnetic adsorbent was also confirmed by applying Cu2+, Ni2+, Pb2+ and Cd2+ ions in adsorption experiments.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Over the last century, there has been an unsustainable increase in human-caused environmental pollution. Our soil, air and water are increasingly polluted (Nica et al. 2019). Heavy metal ions are among the most dangerous contaminants, which can cause serious health problems (Järup 2003). Metals such as arsenic, lead, copper, cadmium, nickel, mercury, chromium, cobalt, and zinc are dangerous not just for humans, but can be harmful to all living organism (Masindi and Muedi 2018). The widespread metal ion pollution forced the European Council to issue a directive (98/83/EC) about the quality requirements and ion concentration limits of drinking water (< 5 µg/L of Cd, < 2 mg/L of Cu, < 20 µg/L of Ni and < 10 µg/L of Pb). Numerous studies have been dealing with the removal of heavy metal ions from water, providing more and better alternatives to solve this ecological problem (Anuja Ashok Bhatt 2015; Carolin et al. 2017; Kobielska et al. 2018; Siyal et al. 2018). The developed technologies are based on various procedures such as membrane filtration (Altalhi et al. 2016), chemical precipitation (Charerntanyarak 1999), ion exchange (Pansini et al. 1991), electrochemical treatment (Janssen and Koene 2002), and adsorption.
Adsorption techniques have been of great interest, because compared with other methods, it has significant advantages including simple design, easy operation, and high separation efficiency (Afroze and Sen 2018). A wide range of systems have been tested as adsorbents, including biopolymers (Huang et al. 1996; Wan Ngah et al. 2011) and waste materials (Orhan and Büyükgüngör 1993) or synthetic materials like ion-exchange resins (Alexandratos 2009). Industrial-scale applications are also known with ion-exchange resin, activated carbon and silica gel (Karnib et al. 2014), but there are promising materials which have only been tested on laboratory scale, i.e., graphene microbots (Vilela et al. 2016), or different nanomaterials (Wang et al. 2012) and nanocomposites (Sharma et al. 2014). There are also many interesting new experiments on the subject, where for example pyrolyzed camel bones (Alqadami et al. 2018) or watermelon rind extract-capped ZnS nanoparticles (Devasangeeth et al. 2018) were used.
Excellent adsorption capacity has been achieved with Amberlite resin for Cu, Zn, Ni, Cd, Pb (Demirbas et al. 2005) and Hg (Naushad et al. 2016). It has been proved with column experiments that the Amberlite IR-120 resin is selective for Pb2+ in quantitative binary separation (Naushad and Alothman 2015). In this work, a synthetic ion-exchange resin-based system has been applied as adsorbent and combined with magnetic properties.
The adsorption capacity of magnetic materials has been investigated in several studies (Ozay et al. 2009)(Ge et al. 2012). The magnetites have also been studied and 8.67 mg/g and 18.61 mg/g adsorption has been reached for Cr and Cu ions, respectively, by using a mixed solution of 80 mg/L Cr(VI) and Cu(II) (Zhang et al. 2020). In a similar study, 9.72 mg/g was measured as the maximum adsorption capacity for arsenic removal (Darezereshki et al. 2018). In another case, the total amount of lead was removed from an aqueous medium by using magnetite (50 mg/L with 20 mg/L adsorbent dose) (Bagbi et al. 2016). In each of these studies, the adsorption capacity of magnetite was a result of the OH groups which were present on the surface and initiated electrostatic interaction with the metal ions. Hu and his colleagues created magnetite nanoparticles for Cr(VI) removal and achieved 11.23 mg/g maximum capacity (Hu et al. 2004). Activated carbon containing magnetic alginate beads (AC-MAB) were tested against activated carbon to compare the adsorption capacities of the systems and it was found that the AC-MAB was > 62 times better adsorbent for methylene blue (MB) (Rocher et al. 2008). Magnetic chitosan/clay beads were also tested for MB dye removal and a maximum uptake of 82 mg/g was achieved (Bée et al. 2017). Magnetite particles are frequently used, because they are biocompatible, inexpensive, chemically stable and their surface modification is easy (Davarpanah et al. 2015; Vojoudi et al. 2017).
The general procedure of heavy metal removal has two steps, first, the precipitation settling of the metal hydroxide is carried out, which is followed by the thickening or filtration of the sludge (Feng et al. 2000). This method has several disadvantages including the potential incomplete precipitation, chemical instability of the precipitates and formation of large volumes of sludge which can be difficult to filter (Feng et al. 2000). These disadvantages can be avoided by applying ion-exchange resins. However, the system filled with ion-exchange resins will initiate backpressure into the columns and limit the flow rate and the processing throughput of industrial wastewater (Zhang et al. 2019). Therefore, a suspended solid adsorbent which can be easily removed from the aqueous medium is preferred. Such adsorbent could be a resin combined with magnetic properties in fluidized beds (Feng et al. 2000). By using a magnetic adsorbent, the separation from the aqueous medium by applying magnetic field is not just easier, but also faster, selective and efficient (Xu et al. 2010; Sharma et al. 2018).
In our work, the excellent adsorption properties of ion-exchange resins with the magnetic properties of magnetite have been combined, using a simple preparation method. Ion-exchange resin beads were applied for magnetite (Fe3O4) synthesis, to create adsorbents (magnetic polymer beads, MPB), which can be separated by magnetic field. The ion-exchange effect of the magnetic adsorbent was confirmed by Cu2+, Ni2+, Pb2+ and Cd2+ ion adsorption experiments.
Experimental
Materials and methods
Iron(III) chloride hexahydrate (VWR), iron(II) sulfate heptahydrate (Reanal) and sodium hydroxide (Sigma Aldrich) were used for magnetite synthesis. Amberlite IR120 hydrogen form ion-exchange resin (ACROS Organics MS, Table 1) was used to create the magnetic polymer beads. The synthesized MPBs have been tested in Cu2+ Ni2+, Pb2+ and Cd2+ adsorption experiments by using copper(II) chloride (Aldrich), nickel(II) nitrate hexahydrate (Reanal), lead(II) nitrate (Reanal) and cadmium(II) nitrate (Reanal). In every experiment, eight diluted samples (0.5–10 mmol/L) were made from the corresponding stock solution (50 mmol/L). Each sample contained 0.2 g magnetic beads, and 50 mL solution with different concentrations. The samples were shaken for 2 h at room temperature on a horizontal shaker. The parameters were selected based on preliminary studies to achieve full adsorption isotherm.
The particle size and morphology were studied by high-resolution scanning electron microscope (SEM) applying a JEOL JSM 7200F instrument, using carbon tape for sample preparation. The diameters of the magnetic particles were measured by using the ImageJ program. BET specific surface measurements were carried out by using N2 with a Micrometics TriStar 3000 instrument. The phase identification of the magnetic iron oxide particles was carried out by X-ray diffraction (XRD) performed with a Rigaku Miniflex II diffractometer with Cu Kα radiation source at 30 kV and 15 mA. The MPB samples were pulverized with a mortar before the measurement. The pure magnetite, the Amberlite IR120 hydrogen form ion-exchange resin and the synthesized MPB were also measured using a Bruker Vertex 70 Fourier-transform infrared spectroscope (FTIR, detection range was 4000–400 cm−1 at 4 cm−1 optical resolution). All samples were studied in potassium bromide pellets (5 mg sample in 250 mg KBr). The concentrations of the heavy metal solutions were further analyzed by a Varian 720 ES inductively coupled optical emission spectrometer (ICP-OES) using Merck Certipur ICP multi-element standard IV. The measurement conditions and parameters are listed in Table 2. The detection limit for Cd, Pb, Ni, and Cu is 0.005 mg/L, 0.04 mg/L, 0.02 mg/L, and 0.04 mg/L, respectively, while the relative standard deviation is < 5%.
Synthesis of the magnetic adsorbents
In first step, the Amberlite IR120 hydrogen form resin was dried at 120 °C for 24 h. Then, the aqueous solution (25 mL) of FeSO4·7H2O (3.06 g) and FeCl3·6H2O (5.40 g) was used for ion exchange. The dried Amberlite resin (7.50 g) was added to the Fe(II)/Fe(III) solution and after 2 h of contact time, the impregnated beads were washed with distilled water (Fig. 1). Sodium hydroxide solution (7 g NaOH/50 mL water) was added to the beads, and after continuous agitation for 1 h, the beads were separated from the alkalic media, washed and dried at 120 °C overnight. Sodium hydroxide has a dual role, on the one hand it facilitates the formation of magnetite by co-precipitation, while on the other hand, it participates in the regeneration of the ion-exchange resin (Fig. 1).
Results and discussion
Analysis of the morphology of the synthesized magnetite particles
The prepared magnetic polymer beads (MPB) have been studied by SEM and their diameter was measured and found to be between 500 and 800 μm (Fig. 2a). The beads are intact, with no breakage, cracking, or damage. The surface of the MPBs are abundantly covered with well-dispersed, irregular hexagonal magnetite crystals (Fig. 2b, c). The size distribution of the magnetite particles’ diameter is also examined (Fig. 3.). The average diameter of the hexagons is ~ 89 nm.
Heavy metal adsorption experiments have been carried out and the SEM analysis have also been performed after this process. There is no significant visible difference between the images taken before or after metal removal (SI Figs. 1–5). It can be seen on the EDS spectra (SI Figs. 1–5) that the MPB sample had high sodium content before adsorption, which decreased after adsorption, and characteristic peaks of the adsorbed metals appeared. This suggests that the heavy metal adsorption mostly happened by ion-exchange process.
Phase analysis of the magnetic iron-oxide
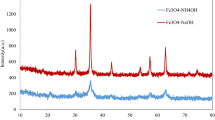
Six stronger diffraction peaks were identified on the XRD spectrum of the powdered MPBs at the 2θ of 30°, 36°, 43°, 53°, 57° and 63° (Fig. 4), which correspond to the (220), (311), (400), (422), (511), and (440) crystalline planes of magnetite phase (Fe3O4), respectively (Kazeminezhad and Mosivand 2014). Two additional weaker peaks have also been found at 33° and 39° 2θ, which can be associated with the (104) and (113) planes and indicates the presence of an α-Fe2O3, hematite phase (Chernyshova et al. 2007).
FTIR measurements of the magnetic adsorbent
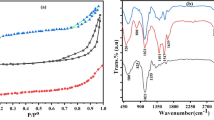
The FTIR spectra of pure Amberlite IR120 hydrogen form resin, magnetite and the final MPB were measured and compared (Fig. 5). The widest band appeared between 3000 and 3700 cm−1 and it can be associated with the vibration of O–H groups in the systems (Fig. 5). It is important that OH groups have been detected on the surface of the magnetite, because their presence could promote adsorption. The aliphatic C–H stretching can also be identified at 2853 cm−1 and 2929 cm−1 (Singare et al. 2011). The presence of C=O groups is indicated by the band at ~ 1630 cm−1 (Singare et al. 2011), while the band at 1158 cm−1 indicates that there are also C–N groups in the samples (Haslaniza et al. 2015). The peaks observed at 675 cm−1, 1040 cm−1, and 1408 cm−1 are assigned to S–O and the stretching of SO3 and O–S–O group, respectively (Jha et al. 2009; Singare et al. 2011). The characteristic absorption band of the Fe–O bond also appeared at 560 cm−1 (Bini et al. 2012). The groups detected in the pure materials (magnetite and Amberlite) can also be found in MPB (Fig. 5, top). There are only minor shifts in the positions of the bands, and new band has not been identified.
Adsorption tests of the synthesized magnetic polymer beads
BET specific surface area (SSA) measurements were also performed on the samples, before and after adsorption. However, in each case, the SSA was below the measurement limit, which suggests that all samples have a BET specific surface area < 1 m2/g.
The heavy metal ion adsorption capacity of the synthesized MPBs was tested using Cu2+, Ni2+, Pb2+ and Cd2+.The Langmuir, the Freundlich and the Redlich–Peterson isotherm models were used to describe the equilibrium characteristics of the adsorption.
The Langmuir is defined by the following equations:
where qe is the amount (mg/g), while ce is the equilibrium concentration of the heavy metal ion in the solution (mg/L), qm is the maximum sorption capacity (mg/g) and KL is the Langmuir equilibrium constant (L/mg). KL and qm can be calculated with nonlinear regression. RL is the separation factor without unit and co is the initial heavy metal concentration (mg/L).
The Freundlich isotherm can be described as follows:
where KF and n are constants.
The Redlich–Peterson (Eq. 4.) is a hybrid isotherm model that combines the Freundlich and the Langmuir models with three parameters (Al-Ghouti and Da’ana 2020):
where KR, aR and b are constants. Previous studies have shown that it is more accurate than the Langmuir and the Freundlich models, because it contains three tuning parameters (Kumara et al. 2014).
For each heavy metal ions, eight dilution points have been applied during the measurements. By comparing the three fitted isotherms (Table 3), it was found that the correlation coefficients (R2) are close to 1 in each case, and the isotherms fit well to the results, but as it was expected, the most accurate is the Redlich–Peterson isotherm. However, the Langmuir model will be used in the following discussion (Fig. 6) as it is the most commonly applied isotherm in similar studies and the difference between the two fits (Langmuir vs Redlich–Peterson) is not significant.
According to the results, the highest qm was obtained in the case of Pb2+ ions (3.64 mmol/g), while the highest affinity (KL) was observed for nickel 0.57 L/mg. The separation factors indicate strong interactions between the heavy metal ions and the MPB adsorbent and show that the isotherm type is favorable (0 < RL < 1) (Kocaoba 2007)(Tan et al. 2012).
Computational and experimental adsorption data shows that the dry resin’s adsorption capacity for Pb2+ and Ni2+ ions is 5.56 meq/g (Carmona et al. 2008), while for Cu2+ and Cd2+ ions it is 5.0 meq/g (Valverde et al. 2002). Thus, by adding magnetite to the system, the adsorption capacity of the resin slightly reduced in case of Cu2+, Ni2+, and Cd2+ ions, but improved for Pb2+. However, the trend in the adsorption capacity Pb2+ > Cu2+ > Ni2 + > Cd2 + is similar to a previous study where pure resin was tested as adsorbent (Naushad and Alothman 2015).
The synthesized MPB’s adsorption parameters and capacity have also been compared to other magnetic adsorbents from the literature (Table 4). It can be seen that the prepared MPB has excellent adsorption capacity, and it is the best in almost every aspect.
After the adsorption tests, the magnetic beads were removed easily from the aqueous media by using a magnet (Fig. 7).
Conclusions
In our work, magnetic polymer beads were successfully created starting from Amberlite IR120 ion-exchange resin beads. The diameter of the magnetic polymer beads is in a range of 500–800 μm. The surface of the polymer beads is covered with well-dispersed, hexagonal magnetite crystals. The presence of magnetite particles was verified by XRD measurements. The heavy metal ion adsorption capacity of the synthesized MPB was tested using Cu2+, Ni2+, Pb2+ and Cd2+. The developed MPB is a very efficient adsorbents, especially in the case of Pb removal. Besides its adsorption capacity, it is easy to handle and due to its magnetic property, it can be easily removed from the medium by applying magnetic field. Furthermore, it has higher adsorption capacity in almost all cases for the studied heavy metal ions compared to other magnetic adsorbents from the literature.
References
Afroze S, Sen TK (2018) A review on heavy metal ions and dye adsorption from water by agricultural solid waste adsorbents. Water Air Soil Pollut 229:225. https://doi.org/10.1007/s11270-018-3869-z
Alexandratos SD (2009) Ion-exchange resins: a retrospective from industrial and engineering chemistry research. Ind Eng Chem Res 48:388–398. https://doi.org/10.1021/ie801242v
Al-Ghouti MA, Da’ana DA (2020) Guidelines for the use and interpretation of adsorption isotherm models: a review. J Hazard Mater 393:122383
Alqadami AA, Khan MA, Otero M et al (2018) A magnetic nanocomposite produced from camel bones for an efficient adsorption of toxic metals from water. J Clean Prod 178:293–304. https://doi.org/10.1016/J.JCLEPRO.2018.01.023
Altalhi T, Mezni A, Aldalbahi A et al (2016) Fabrication and characterisation of sulfur and phosphorus (S/P) co-doped carbon nanotubes. Chem Phys Lett 658:92–96. https://doi.org/10.1016/j.cplett.2016.06.028
Anuja Ashok Bhatt MK (2015) Removal of heavy metals from water (Cu and Pb) using household waste as an adsorbent. J Bioremed Biodegrad. https://doi.org/10.4172/2155-6199.1000269
Bagbi Y, Sarswat A, Mohan D et al (2016) Lead (Pb2+) adsorption by monodispersed magnetite nanoparticles: surface analysis and effects of solution chemistry. J Environ Chem Eng 4:4237–4247. https://doi.org/10.1016/j.jece.2016.09.026
Bée A, Obeid L, Mbolantenaina R et al (2017) Magnetic chitosan/clay beads: a magsorbent for the removal of cationic dye from water. J Magn Magn Mater 421:59–64. https://doi.org/10.1016/J.JMMM.2016.07.022
Bini RA, Marques RFC, Santos FJ et al (2012) Synthesis and functionalization of magnetite nanoparticles with different amino-functional alkoxysilanes. J Magn Magn Mater 324:534–539. https://doi.org/10.1016/J.JMMM.2011.08.035
Carmona M, Warchoł J, De Lucas A, Rodriguez JF (2008) Ion-exchange equilibria of Pb2+, Ni2+, and Cr3+ ions for H+ on amberlite IR-120 resin. J Chem Eng Data 53:1325–1331. https://doi.org/10.1021/je8000552
Carolin CF, Kumar PS, Saravanan A et al (2017) Efficient techniques for the removal of toxic heavy metals from aquatic environment: a review. J Environ Chem Eng 5:2782–2799. https://doi.org/10.1016/j.jece.2017.05.029
Charerntanyarak L (1999) Heavy metals removal by chemical coagulation and precipitation. Water Sci Technol 39:135–138. https://doi.org/10.1016/S0273-1223(99)00304-2
Chernyshova IV, Hochella MF Jr, Madden AS (2007) Size-dependent structural transformations of hematite nanoparticles. 1. Phase transition. Phys Chem Chem Phys 9:1736. https://doi.org/10.1039/b618790k
Darezereshki E, Darban AK, Abdollahy M, Jamshidi-Zanjani A (2018) Influence of heavy metals on the adsorption of arsenate by magnetite nanoparticles: kinetics and thermodynamic. Environ Nanotechnol Monit Manag 10:51–62. https://doi.org/10.1016/j.enmm.2018.04.002
Davarpanah M, Ahmadpour A, Rohani Bastami T (2015) Preparation and characterization of anion exchange resin decorated with magnetite nanoparticles for removal of p-toluic acid from aqueous solution. J Magn Magn Mater 375:177–183. https://doi.org/10.1016/j.jmmm.2014.09.065
Demirbas A, Pehlivan E, Gode F et al (2005) Adsorption of Cu(II), Zn(II), Ni(II), Pb(II), and Cd(II) from aqueous solution on Amberlite IR-120 synthetic resin. J Colloid Interface Sci 282:20–25. https://doi.org/10.1016/J.JCIS.2004.08.147
Devasangeeth SD, Balaji GL, Lakshmipathy R (2018) Multi metal ion sorption capacity of watermelon rind extract capped ZnS nanoparticles. Int J Pure Appl Math 118:1–12
Feng D, Aldrich C, Tan H (2000) Removal of heavy metal ions by carrier magnetic separation of adsorptive particulates. Hydrometallurgy 56:359–368. https://doi.org/10.1016/S0304-386X(00)00085-2
Ge F, Li M-M, Ye H, Zhao B-X (2012) Effective removal of heavy metal ions Cd2+, Zn2+, Pb2+, Cu2+ from aqueous solution by polymer-modified magnetic nanoparticles. J Hazard Mater 211–212:366–372. https://doi.org/10.1016/J.JHAZMAT.2011.12.013
Guo X, Du B, Wei Q et al (2014) Synthesis of amino functionalized magnetic graphenes composite material and its application to remove Cr(VI), Pb(II), Hg(II), Cd(II) and Ni(II) from contaminated water. J Hazard Mater 278:211–220. https://doi.org/10.1016/j.jhazmat.2014.05.075
Haslaniza H, Wan Yaacob WA, Zubairi SI, Maskat MY (2015) Potential of amberlite IRA 67 resin for deacidification of organic acids in noni juice. Der Pharma Chem 7:62–69
Hu J, Lo IMC, Chen G (2004) Removal of Cr(VI) by magnetite. Water Sci Technol 50:139–146. https://doi.org/10.2166/wst.2004.0706
Huang C, Chung Y-C, Liou M-R (1996) Adsorption of Cu(II) and Ni(II) by pelletized biopolymer. J Hazard Mater 45:265–277. https://doi.org/10.1016/0304-3894(95)00096-8
Janssen LJ, Koene L (2002) The role of electrochemistry and electrochemical technology in environmental protection. Chem Eng J 85:137–146. https://doi.org/10.1016/S1385-8947(01)00218-2
Järup L (2003) Hazards of heavy metal contamination. Br Med Bull 68:167–182. https://doi.org/10.1093/bmb/ldg032
Jha MK, Van Nguyen N, Lee-chun J et al (2009) Adsorption of copper from the sulphate solution of low copper contents using the cationic resin Amberlite IR 120. J Hazard Mater 164:948–953. https://doi.org/10.1016/j.jhazmat.2008.08.103
Karnib M, Kabbani A, Holail H, Olama Z (2014) Heavy metals removal using activated carbon, silica and silica activated carbon composite. Energy procedia. Elsevier Ltd, Amsterdam, pp 113–120
Kazeminezhad I, Mosivand S (2014) Phase transition of electrooxidized Fe3O4 to γ and α-Fe2O3 nanoparticles using sintering treatment. Acta Phys Pol A 125:1210–1214. https://doi.org/10.12693/APhysPolA.125.1210
Kobielska PA, Howarth AJ, Farha OK, Nayak S (2018) Metal–organic frameworks for heavy metal removal from water. Coord Chem Rev 358:92–107. https://doi.org/10.1016/J.CCR.2017.12.010
Kocaoba S (2007) Comparison of Amberlite IR 120 and dolomite’s performances for removal of heavy metals. J Hazard Mater 147:488–496. https://doi.org/10.1016/j.jhazmat.2007.01.037
Kumara NTRN, Hamdan N, Petra MI et al (2014) Equilibrium isotherm studies of adsorption of pigments extracted from Kuduk-kuduk (Melastoma malabathricum L.) pulp onto TiO2 nanoparticles. J Chem. https://doi.org/10.1155/2014/468975
Masindi V, Muedi KL (2018) Environmental Contamination by heavy metals. In: Heavy Metals. InTech
Naushad M, Alothman ZA (2015) Separation of toxic Pb2+ metal from aqueous solution using strongly acidic cation-exchange resin: analytical applications for the removal of metal ions from pharmaceutical formulation. Desalin Water Treat 53:2158–2166. https://doi.org/10.1080/19443994.2013.862744
Naushad M, Vasudevan S, Sharma G et al (2016) Adsorption kinetics, isotherms, and thermodynamic studies for Hg2+ adsorption from aqueous medium using alizarin red-S-loaded amberlite IRA-400 resin. Desalin Water Treat 57:18551–18559. https://doi.org/10.1080/19443994.2015.1090914
Nica A, Popescu A, Ibanescu D-C (2019) Human influence on the climate system. Curr Trends Nat Sci 8:209–215
Orhan Y, Büyükgüngör H (1993) The removal of heavy metals by using agricultural wastes. Water Sci Technol 28:247–255. https://doi.org/10.2166/wst.1993.0114
Ozay O, Ekici S, Baran Y et al (2009) Removal of toxic metal ions with magnetic hydrogels. Water Res 43:4403–4411. https://doi.org/10.1016/J.WATRES.2009.06.058
Pansini M, Colella C, De Gennaro M (1991) Chromium removal from water by ion exchange using zeolite. Desalination 83:145–157. https://doi.org/10.1016/0011-9164(91)85091-8
Rocher V, Siaugue J-M, Cabuil V, Bee A (2008) Removal of organic dyes by magnetic alginate beads. Water Res 42:1290–1298. https://doi.org/10.1016/j.watres.2007.09.024
Sharma G, Pathania D, Naushad M, Kothiyal NC (2014) Fabrication, characterization and antimicrobial activity of polyaniline Th(IV) tungstomolybdophosphate nanocomposite material: efficient removal of toxic metal ions from water. Chem Eng J 251:413–421. https://doi.org/10.1016/j.cej.2014.04.074
Sharma M, Kalita P, Senapati KK, Garg A (2018) Study on magnetic materials for removal of water pollutants. In: Emerging pollutants—some strategies for the quality preservation of our environment. InTech
Singare PU, Lokhande RS, Madyal RS (2011) Thermal degradation studies of some strongly acidic cation exchange resins. Open J Phys Chem 1:45–54. https://doi.org/10.4236/ojpc.2011.12007
Siyal AA, Shamsuddin MR, Khan MI et al (2018) A review on geopolymers as emerging materials for the adsorption of heavy metals and dyes. J Environ Manag 224:327–339. https://doi.org/10.1016/J.JENVMAN.2018.07.046
Son EB, Poo KM, Mohamed HO et al (2018) A novel approach to developing a reusable marine macro-algae adsorbent with chitosan and ferric oxide for simultaneous efficient heavy metal removal and easy magnetic separation. Bioresour Technol 259:381–387. https://doi.org/10.1016/j.biortech.2018.03.077
Tan Y, Chen M, Hao Y (2012) High efficient removal of Pb(II) by amino-functionalized Fe3O4 magnetic nano-particles. Chem Eng J 191:104–111. https://doi.org/10.1016/j.cej.2012.02.075
Valverde JL, De Lucas A, González M, Rodríguez JF (2002) Equilibrium data for the exchange of Cu2+, Cd2+, and Zn2+ ions for H+ on the cationic exchanger Amberlite IR-120. J Chem Eng Data 47:613–617. https://doi.org/10.1021/je010299g
Vilela D, Parmar J, Zeng Y et al (2016) Graphene-based microbots for toxic heavy metal removal and recovery from water. Nano Lett 16:2860–2866. https://doi.org/10.1021/acs.nanolett.6b00768
Vojoudi H, Badiei A, Bahar S et al (2017) A new nano-sorbent for fast and efficient removal of heavy metals from aqueous solutions based on modification of magnetic mesoporous silica nanospheres. J Magn Magn Mater 441:193–203. https://doi.org/10.1016/J.JMMM.2017.05.065
Wan Ngah WS, Teong LC, Hanafiah MAKM (2011) Adsorption of dyes and heavy metal ions by chitosan composites: a review. Carbohydr Polym 83:1446–1456. https://doi.org/10.1016/j.carbpol.2010.11.004
Wang X, Gou Y, Yang L et al (2012) Nanomaterials as sorbents to remove heavy metal ions in wastewater treatment. J Environ Anal Toxicol. https://doi.org/10.4172/2161-0525.1000154
Xu Y, Chen L, Wang H et al (2010) Preparation of magnetic strong cation exchange resin for the extraction of melamine from egg samples followed by liquid chromatography-tandem mass spectrometry. Anal Chim Acta 661:35–41. https://doi.org/10.1016/j.aca.2009.12.002
Zhang T, Dai S, Mo S et al (2019) Magnetic ion-exchange resins for superconducting magnetic separation system: kinetics, isotherm, and mechanism of heavy metals removal magnetic ion-exchange resins for superconducting magnetic separation system: kinetics, isotherm, and mechanism of. Mater Sci Eng. https://doi.org/10.1088/1757-899X/490/2/022015
Zhang J, Lin S, Han M et al (2020) Adsorption properties of magnetic magnetite nanoparticle for coexistent Cr(VI) and Cu(II) in mixed solution. Water 12:446. https://doi.org/10.3390/w12020446
Acknowledgements
We are grateful to JEOL (EUROPE) SAS for the SEM images, and to Gertrúd Oszkó who helped with communication issues. ES was supported by the ÚNKP-19-3 New National Excellence Program of the Ministry for Innovation and Technology. This research was supported by the European Union and the Hungarian State, co-financed by the European Regional Development Fund in the framework of the GINOP-2.3.4-15-2016-00004 project, aimed to promote the cooperation between the higher education and the industry.
Funding
Open access funding provided by University of Miskolc.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Sikora, E., Hajdu, V., Muránszky, G. et al. Application of ion-exchange resin beads to produce magnetic adsorbents. Chem. Pap. 75, 1187–1195 (2021). https://doi.org/10.1007/s11696-020-01376-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11696-020-01376-y