Abstract
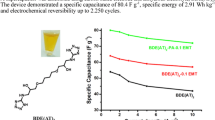
Binderless chemical synthesis of flexible electrodes (FEs) of FeO(OH) has been carried out by simple and cost-effective successive ionic layer adsorption and reaction (SILAR) technique. In aqueous route synthesis, FeCl3 and NaOH solutions were used as cationic and anionic sources, respectively. Molar concentration of NaOH was kept constant while the molar concentration of FeCl3 was varied from 0.2 to 0.8 M with step increments of 0.2 M to study its effect on physical and electrochemical characteristics of prepared FEs. X-ray diffraction (XRD) patterns of the FEs exhibit existence of tetragonal FeO(OH). SEM images show the rice grain-like morphologies. Cyclic voltammetric analyses indicate the decrease in specific capacitance value with the increase in molar concentration of cationic precursor. For the electrode prepared with 0.2 M FeCl3 as precursor, the observed maximum specific capacitance (SC) was 444.44 F g−1 at 5 mV s−1 in 1 M NaOH, hence these electrodes were used to fabricate the symmetric solid state supercapacitors. Prepared symmetric supercapacitive devices (SSD) were electrochemically analyzed. The maximum SC for the symmetric supercapacitor was found to be 320.50 F g−1 at 5 mV s−1 which was nearly same as that given by GCD analysis which is 313.27 F g−1 at 0.5 mA. Nyquist plot of the device shows minute semicircle in the high frequency region and the mid-low frequency region shows straight line with inclination of nearly \( 40^\circ \) with X-axis. The equivalent series resistance (ESR), charge transfer resistance (Rct) and Warburg impedance (Rw) are found to be 2.58, 2.56 and 0.8 Ω, respectively. The prepared SSD shows high cycling stability with 88% of capacitive retention even after 2000 cycles.



Similar content being viewed by others
Abbreviations
- Ω :
-
Unit of resistance (ohm)
- Μm:
-
Micrometer (10−6 m)
- Z′ :
-
Real impedance in ohm (ohm
- Z′′ :
-
Imaginary impedance in ohm (ohm
- T :
-
Time (S)
- m:
-
Mass (G)
- I d :
-
Discharging current (A)
- t d :
-
Discharging time (S)
- t c :
-
Charging time (S)
- V :
-
Potential (volt)
- M:
-
Molarity (mol)
- ESR:
-
Equivalent series resistance (ohm)
- R ct :
-
Charge transfer resistance (ohm)
- R w :
-
Warburg impedance (ohm)
- SC:
-
Specific capacitance (ohm)
- Ω :
-
Unit of resistance (Ohm)
- Μm:
-
Unit of length/thickness (10−6 m)
References
Al-osta A, Jadhav VV, Zate MK, Mane RS, Hui KN, Han SH (2015), Electrochemical supercapacitors for anodized brass templated NiO nanostructured electrodes Scr. Mater 99: 29. https://doi.org/10.1016/j.scriptamat.2014.11.019
Ansari SA, Fouad H, Ansari SG, Sk MP, Cho MH (2017) Mechanically exfoliated MoS2sheet coupled with conductive polyaniline as a superior supercapacitor electrode material. J Colloid Interface Sci. https://doi.org/10.1016/j.jcis.2017.05.064
Barik A, Mohapatra M (2015) α Fe2O3 nanomaterials: facet sensitive energy storage materials. Cryst. Eng. Comm. 17(47):9203–9215. https://doi.org/10.1039/C5CE01369K
Choudhari S, Bhattacharya D, Jong-sung Y (2013) 1-Dimensional porous α Fe2O3 nanorods as high performance electrode material for supercapacitor. RSC Adv 3:25120. https://doi.org/10.1039/c3ra44159h
Conway BE (1999) Electrochemical supercapacitors scientific fundamentals and technological applications. Kluwer-Plenum, New York. ISBN 978-1-4757-3058-6
Deshmukh PR, Pusawale SN, Jagadale AD, Lokhande CD (2012) Supercapacitive performance of hydrous ruthenium oxide (RuO2·nH2O) thin films deposited by SILAR method. J Mater Sci 7(3):1546–1553. https://doi.org/10.1007/s10853-011-5946-1
Dubal DP, Holze R, Gomez-Romero P (2014), Development of hybrid materials based on sponge supported graphene oxide and transition metal hydroxides for hybrid energy storage devices. Sci Rep 4: 7349
Fugare BY, Lokhande BJ (2017) The influence of concentration on the morphology of TiO2 thin films prepared by spray pyrolysis for electrochemical study. Appl Phys A 123:1–10. https://doi.org/10.1007/s00339-017-1008-0
Hou Y, Cheng Y, Hobson T, Liu J (2010), Design and synthesis of hierarchical MnO2 nanospheres/carbon nanotubes/ conducting polymer ternary composite for high performance electrochemical electrodes. Nano Lett 10: 2727–2733. https://doi.org/10.1021/nl101723g
Ingole RS, Lokhande BJ (2015) Effect of pyrolysis temperature on structural, morphological and electrochemical properties of vanadium oxide thin films. Appl Surf Sci 349:887–896
Ingole RS, Lokhande BJ (2016) Spray pyrolyzed vanadium oxide thin films using different ingredients for redox supercapacitors. J Mater Sci Mater Electron 27(2):1363–1369
Jadhav VV, Shinde DV, Patil SA, Zate MK, Osta S, Osta A, Mane RS, Han SH (2014) , Electrochemical performance of anodized copper hydroxide nanostructures. J Nano Engineer Manufact 4(2):168–172
Kim H, Popov BN (2003) Synthesis and characterization of MnO2 based mixed oxides as supercapacitors. J Electrochem Soc 150:D56
Kore RM, Mane RS, Noushad M, Khan MR, Lokhande BJ (2016) Nanomorphology dependent pseudocapacitive properties of NiO electrodes engineered through a controlled potentiodynamic electrodeposition process. RSC Adv. 6:24478
Liu L, Zheng M, Shi X, Zeng H, Xia H (2015) Amorphous FeOOH quantum dots assembled mesoporous film anchored on graphene nanosheets with superior electrochemical performance for supercapacitors. Funct Mater, Adv. https://doi.org/10.1002/adfm.201504019
Mckeown D, Hagans P, Carette L, Russell A, Swider K, Rolison D (1999) Structure of hydrous ruthenium oxides: implications for charge storage. J Phys Chem B 103:4825–4832
Parveen N, Ansari SA, Ansari MO, Cho MH (2017a) Manganese dioxide nanorods intercalated reduced graphene oxide nanocomposite toward high performance electrochemical supercapacitive electrode materials. J Colloid Interface Sci. https://doi.org/10.1016/j.jcis.2017.07.087
Parveen N, Ansari SA, Ansari SA, Fouad H and Cho MH (2017), Intercalated reduced graphene oxide and its content effect on the supercapacitance performance of three dimensional flower like B Ni(OH) architecture. N J Chem https://doi.org/10.1039/c7nj01915g
Shen B, Guo R, Lang J, Li Liu, Liu L, Yan X (2016) A high-temperature flexible supercapacitor based on pseudocapacitive behavior of FeOOH in an ionic liquid electrolyte. J Mater Chem A 4:8316. https://doi.org/10.1039/c6ta01734g
Thakur AV, Lokhande BJ (2016) Effect of dip time on the electrochemical behavior of PPy-Cu(OH)2 hybrid electrodes synthesized using pyrrole and Cu(SO)4. e-Polymer. https://doi.org/10.1515/epoly-2016-0160
Thakur AV, Lokhande BJ (2017a) Dip time dependent SILAR synthesis and electrochemical study of highly flexible PPy-Cu(OH)2 hybrid electrodes for supercapacitors. J Solid State Electrochem. https://doi.org/10.1007/s10008-016-3502-2
Thakur AV, Lokhande BJ (2017b) Electrolytic anion affected charge storage mechanisms of Fe3O4 flexible thin film electrode in KCl and KOH: a comparative study by cyclic voltammetry and galvanostatic charge–discharge. Mater Electron, J Mater Sci. https://doi.org/10.1007/s10854-017-6980-9
Thakur AV, Lokhande BJ (2017c) C10H8N2-PPy hybrid flexible electrodes: SILAR synthesis and electrochemical study, J. Mater Electron, Mater Sci. https://doi.org/10.1007/s10854-017-8074-0
Wang G, Zhang L, Zhang J (2012) A review on material for electrochemical supercapacitors. Chem Soc Rev 41:797
Xu H et al (2013) Synthesis and super capacitance of goethite/reduced graphene oxide for supercapacitors. Mater Chem Phys. https://doi.org/10.1016/j.matchemphys.2013.04.048
Xu Y, Ding L, Zhong T, Han X, Jiao L, Yuan H, Wang Y (2015), Novel application of LiCoO as high performance candidate material for supercapacitor. J Energy Chem 24:193–198
Zhao X, Sanchez M, Dobson P, Grant P (2011), The role of nanomaterials in redox based supercapacitors for next generaation nergy storage devices, Nanoscale 3:839
Zhong J, Wang A, Li G, Wang J, Ou Y and Tong Y (2012), Co o /Ni(OH) composite mesoporus nanosheet networks as a promising electrode for supercapacitor applications. J Mater Chem 22: 5656-5665
Zhou X, Shen X, Xia Z, Zhang Z, Li J, Ma Y, Qu Y (2015), Hollow fluffy Co3o4 cages as efficient electroactive materials for supercapacitors and oxygen evolution reaction. ACS Appl. Mat Interfaces 7:20322
Acknowledgements
Authors are thankful to Solapur University, Solapur for providing the DRF facility.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Thakur, A.V., Lokhande, B.J. Source molarity affected surface morphological and electrochemical transitions in binder-free FeO(OH) flexible electrodes and fabrication of symmetric supercapacitive device. Chem. Pap. 72, 1407–1415 (2018). https://doi.org/10.1007/s11696-018-0383-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11696-018-0383-0