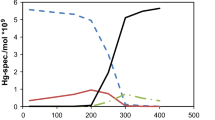
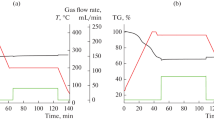
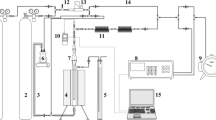
Abstract
Sorption of hydrogen chloride gas by active soda and that of hydrogen sulfide gas by calcium oxide are explored by experiment as promising means of removing these detrimental contaminants from fuel gas: active Na2CO3 was prepared by the calcination of commercial NaHCO3 at 200 °C; reactive CaO was formed by decomposing a fine-grained, high-calcium limestone at 830 °C. Techniques with a differential reactor were employed to explore the rate of reaction of HCl with Na2CO3 at 500 °C and that of H2S with CaO at 800 °C. Time-resolved data on the sorbents’ conversion were collected as a function of mean particle size in the range between 0.285 and 1.12 mm. The surface reaction constants, deduced via the tractable model from the initial reaction rates of the two reactions, slightly increase with the increasing particle size. The proposed correlations enable to interpolate or cautiously extrapolate to other isotropic, irregularly shaped solids. The effective diffusivities educed by means of the model from the experimental curves decrease significantly with the increasing conversion and are affected by the particle size in both sorptions. The developed reaction rate equations can conveniently be applied to the design and simulation of the deep dechloridization and the bulk desulfurization of hot producer gas.


















Similar content being viewed by others
Abbreviations
- D :
-
Effective (apparent) diffusion (transport) coefficient of reactant gas through the product shell, cm2 s−1
- d p :
-
Particle diameter, cm
- \( \bar{d}_{\text{p}} \) :
-
Mean particle size determined by sieving, cm
- (dr′/dτ′):
-
Dimensionless rate of reaction defined by Eq. (23)
- (dX/dτ):
-
Rate of reaction defined by Eq. (24), s−1
- (dX/dτ)in :
-
Initial rate of reaction (for τ → 0 and X → 0) (\( = \)3Kyρg /(r pρp)), s−1
- (dX/dτ′):
-
Dimensionless rate of reaction defined by Eq. (24)
- e :
-
Dimensionless fractional porosity
- e in :
-
Dimensionless initial fractional porosity of a particle
- e x :
-
Dimensionless fractional porosity of a reacted particle
- f :
-
Relative increase or decrease in the solid volume caused by chemical reaction
- F :
-
Specific reaction surface area (\( = \)3r′2/r p \( = \) 3(1 − X)2/3/r p), cm−1
- K :
-
Effective reaction rate constant per unit area of reaction interface (\( = \)[r pρp/(3yρg)]·(dX/dτ)in \( = \) (r pρp)/(τstd yρg)]), cm s−1
- K HCl :
-
Dimensionless equilibrium constant for reaction (1a)
- K H2S :
-
Equilibrium constant for reaction (1), kPa
- N :
-
Rate of disappearance of gas reactant in unit volume of sorbent particle volumetric rate of reaction defined by Eq. (32), mol cm−3 s−1
- P :
-
Total pressure in the main stream, kPa
- p :
-
Dimensionless auxiliary function defined by Eq. (15)
- p i :
-
Partial pressure of species, kPa
- q :
-
Dimensionless auxiliary function defined by Eq. (16)
- R 2 :
-
Dimensionless regression parameter
- r :
-
Radius of reaction interface within a spherical particle, cm
- r p :
-
Radius of a spherical particle, cm
- r′:
-
Relative radius of reaction interface within a spherical particle (\( = \) r/r p \( = \)(1 − X)1/3)
- t :
-
Celsius temperature, °C
- T :
-
Thermodynamic temperature, K
- V i :
-
Molar volume of solid component i, cm3 mol−1
- X :
-
Dimensionless fractional conversion of solid reactant (\( = \)1 − r′3)
- y i :
-
Mole fraction of species
- ΔH°:
-
Standard enthalpy change of reaction, kJ mol−1
- λ :
-
Dimensionless ratio of diffusional resistance in product shell to chemical reaction resistance at interface (\( = \) Kr p/D)
- π :
-
Dimensionless Ludolf constant (\( = \)3.141593)
- ρ g :
-
Molar density of gas at ambient pressure (101.325 kPa) (\( = \)0.012187/T), mol cm−3
- ρp :
-
Particle (apparent) molar density of solid, mol cm−3
- τ :
-
Elapsed time of exposure of solid to reactant gas, s
- τ std :
-
Standard time of exposure of solid to reactant gas (\( = \) r pρp /(Kyρg) \( = \) r 2p ρp /(λDyρg) \( = \) 3/(dX/dτ)in), s
- τ′:
-
Relative time of sorption / reaction (\( = \)τ/τstd \( = \) Kyρgτ /(r pρp) \( = \) λDyρgτ /(r 2p ρp) \( = \) τ d′ + τr′)
- τd′:
-
Relative time of exposure corresponding to diffusional resistance alone (\( = \) λ(2r′3 − 3r′2 + 1)/6)
- τr´:
-
Relative time of exposure corresponding to surface reaction alone (\( = \)1 − r′)
- cos−1 :
-
Anti-cosine; angle whose cosine is given, rad
- | |:
-
Absolute (positive) value
References
Aravind PV, de Jong W (2012) Evaluation of high temperature gas cleaning options for biomass gasification product gas for solid oxide fuel cells. Prog Energy Combust Sci 38:737–764. doi:10.1016/j.pecs.2012.03.006
Barin I (1995) Thermochemical data of pure substances, 3rd edn. VCH, Weinheim
Borgwardt RH (1989a) Sintering of nascent calcium oxide. Chem Eng Sci 44:53–60. doi:10.1016/0009-2509(89)85232-7
Borgwardt RH (1989b) Calcium oxide sintering in atmospheres containing water and carbon dioxide. Ind Eng Chem Res 28:493–500. doi:10.1021/ie00088a019
Borgwardt RH, Roache NF (1984) Reaction of H2S and sulfur with limestone particles. Ind Eng Chem Proc Des Develop 23:742–748. doi:10.1021/i200027a020
Borgwardt RH, Roache NF, Bruce KR (1984) Surface area of calcium oxide and kinetics of calcium sulfide formation. Environ Prog 3(2):129–135. doi:10.1002/ep.670030215
Cigolotti V, McPhail S, Moreno A (2009) Molten carbonate fuel cells fed with biogas: combating H2S. J Fuel Cell Sci Technol 6:3111–3118. doi:10.1016/j.wasman.2010.02.022
Duo W, Seville JPK, Kirkby NF, Clift R (1994) Formation of product layers in solid-gas reactions for removal of acid gases. Chem Eng Sci 49:4429–4442. doi:10.1016/S0009-2509(05)80031-4
Duo W, Kirkby NF, Seville JPK, Kiel JHA, Bos A, Den Uil H (1996) Kinetics of HCl reactions with calcium and sodium sorbents for IGCC fuel gas cleaning. Chem Eng Sci 51:2541–2546. doi:10.1016/0009-2509(96)00111-X
Efthimiadis EA, Sotirchos SV (1992) Sulfidation of limestone-derived calcines. Ind Eng Chem Res 31:2311–2321. doi:10.1021/ie00010a009
Erdös E (1967) Mathematical treatment of the heterogenous fixed-bed reactor. Collect Czech Chem Commun 32:1653–1664
Fenouil LA, Lynn S (1996) Design of entrained-flow and moving-, packed-, and fluidized-bed sorption systems: grain-model kinetics for hot coal-gas desulfurization with limestone. Ind Eng Chem Res 35:1024–1043. doi:10.1021/ie950245y
Hartman M, Coughlin RW (1976) Reaction of sulfur dioxide with limestone and the grain model. Am Inst Chem Eng J 22:490–498. doi:10.1002/aic.690220312
Hartman M, Trnka O (1980) Influence of temperature on the reactivity of limestone particles with sulfur oxide. Chem Eng Sci 35:1189–1194. doi:10.1016/0009-2509(80)85109-8
Hartman M, Trnka O (1993) Reactions between calcium oxide and flue gas containing sulfur dioxide at lower temperatures. Am Inst Chem Eng J 39:615–624. doi:10.1002/aic.690390410
Hartman M, Trnka O (2002) Comments on proposal for a regenerative high-temperature process for coal gas clean up with calcined limestone. Ind Eng Chem Res 41:6207–6208. doi:10.1021/ie020193u
Hartman M, Trnka O (2006) Comments on ceria-zirconia high-temperature sorbents. Ind Eng Chem Res 45:1548–1549. doi:10.1021/ie058078h
Hartman M, Svoboda K, Trnka O, Veselý V (1988) Reaction of sulfur dioxide with magnesia in a fluidized bed. Chem Eng Sci 43:2045–2050. doi:10.1016/0009-2509(88)87082-9
Hartman M, Svoboda K, Trnka O, Čermák Ji (2002) Reaction between hydrogen sulfide and limestone calcines. Ind Eng Chem Res 41:2392–2398. doi:10.1021/ie010805v
Hartman M, Svoboda K, Pohořelý M, Šyc M, Jeremiáš M (2013a) Attrition of dolomitic lime in a fluidized-bed reactor at high temperatures. Chem Pap 67:164–172. doi:10.2478/s11696-012-0267-7
Hartman M, Svoboda K, Pohořelý M, Šyc M (2013b) Thermal decomposition of sodium hydrogen carbonate and textural features of its calcines. Ind Eng Chem Res 52:10619–10626. doi:10.1021/ie400896c
Hartman M, Svoboda K, Pohořelý M, Šyc M, Skoblia S, Chen P-Ch (2014) Reaction of hydrogen chloride gas with sodium carbonate and its deep removal in a fixed-bed reactor. Ind Eng Chem Res 53:19145–19158. doi:10.1021/ie503480k
Heesink ABM, Van Swaaij WPM (1995) The sulfidation of calcined limestone with hydrogen sulfide and carbonyl sulfide. Chem Eng Sci 50:2983–2996. doi:10.1016/0009-2509(95)91133-J
Jeremiáš M, Pohořelý M, Bode P, Beňo Z, Svoboda K (2014) Ammonia yield from gasification of biomass and coal in fluidized bed reactor. Fuel 117:917–925. doi:10.1016/j.fuel.2013.10.009
Levenspiel O (1999) Chemical reaction engineering, 3rd edn. Wiley, New York. doi:10.1021/ie990488g
Levenspiel O (2013) The chemical reactor Omnibook, 6th edn. lulu. Com, Corvallis
Lide DR (2010) Handbook of chemistry and physics, 90th edn. CRC Press, Boca Raton
Mocek K, Lippert E, Erdös E (1983) Reactivity of the solid sodium carbonate towards the gaseous hydrogen chloride and the sulfur dioxide. Collect Czech Chem Commun 48:3500–3507. doi:10.1135/cccc19833500
Pohořelý M, Jeremiáš M, Svoboda K, Kameníková P, Skoblia S, Beňo Z (2014) CO2 as moderator for biomass gasification. Fuel 117:198–205. doi:10.1016/j.fuel.2013.09.068
Speight JG (2005) Lange’s handbook of chemistry, 16th edn. McGraw-Hill, New York. doi:10.1036/0071432205
Stemmler M, Müller M (2011) Chemical hot gas cleaning concept for the “Chrisgas” process. Biomass Bioenergy 35:S 105–S 115. doi:10.1016/j.biombioe.2011.03.044
Svoboda K, Pohořelý M, Hartman M, Martinec J (2009) Pretreatment and feeding of biomass for pressurized entrained flow gasification. Fuel Process Technol 90:629–635. doi:10.1016/j.fuproc.2008.12.005
Svoboda K, Pohořelý M, Jeremiáš M, Kameníková P, Hartman M, Skoblja S, Šyc M (2012) Fluidized-bed gasification of coal-oil and coal-water-oil slurries by oxygen-steam and oxygen-CO2 mixtures. Fuel Process Technol 95:16–26. doi:10.1016/j.fuproc.2011.11.001
Trnka O, Hartman M, Beran Z, Svoboda K (1996) Solving reaction models for the systems with particulate solids. Chem Pap 50:167–176
Trnka O, Hartman M, Svoboda K (1997) An alternative semi-implicit Euler method for the integration of highly stiff nonlinear differential equations. Comput Chem Eng 21:277–282. doi:10.1016/S0098-1354(95)00266-9
Verdone N, De Filippis P (2006) Reaction kinetics of hydrogen chloride with sodium carbonate. Chem Eng Sci 61:7487–7496. doi:10.1016/j.ces.2006.08.023
Wen CY (1968) Noncatalytic heterogenous solid fluid reaction models. Ind Eng Chem 60:34–54. doi:10.1021/ie50705a007
Yi KB, Podlaha EJ, Harrison DP (2005) Ceria-zirconia high temperature desulfurization sorbents. Ind Eng Chem Res 44:7086–7091. doi:10.1021/ie050441x
Acknowledgements
This study was supported by the Grant Agency of the Czech Republic (GAČR) through the bilateral grant project of GAČR and National Science Council (NSC) Taiwan, Reg. No. (in ČR) 14-09692J (Reg. No. of foreign Project 102WBS0300011) and Reg. No. (in Taiwan) NSC 103-2923-E-042A-001-MY3. Special thanks are due to Mrs. Eva Fišerová for her dedicated assistance with the manuscript.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Hartman, M., Svoboda, K., Pohořelý, M. et al. Reaction and transport effects in the heterogeneous systems for lean gas purification. Chem. Pap. 71, 563–577 (2017). https://doi.org/10.1007/s11696-016-0038-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11696-016-0038-y