Abstract
Purpose
Roux-en-Y gastric bypass (RYGB) leads to the improvement of many obesity-associated conditions. The degree to which post-operative macronutrient composition contributes to metabolic improvement after RYGB is understudied.
Methods
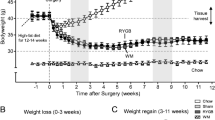
A mouse model of RYGB was used to examine the effects of diet on the post-operative outcomes of RYGB. Obese mice underwent either Sham or RYGB surgery and were administered either chow or HFD and then monitored for an additional 8 weeks.
Results
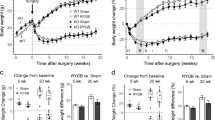
After RYGB, reductions to body weight, fat mass, and lean mass were similar regardless of diet. RYGB and HFD were independently detrimental to bone mineral density and plasma vitamin D levels. Independent of surgery, HFD accelerated hematopoietic stem and progenitor cell proliferation and differentiation and exhibited greater myeloid lineage commitment. Independent of diet, systemic iron deficiency was present after RYGB. In both Sham and RYGB groups, HFD increased energy expenditure. RYGB increased fecal energy loss, and HFD after RYGB increased fecal lipid content. RYGB lowered fasting glucose and liver glycogen levels but HFD had an opposing effect. Indices of insulin sensitivity improved independent of diet. HFD impaired improvements to dyslipidemia, NAFLD, and fibrosis.
Conclusion
Post-operative diet plays a significant role in determining the degree to which RYGB reverses obesity-induced metabolic abnormalities such as hyperglycemia, dyslipidemia, and NAFLD. Diet composition may be targeted in order to assist in the treatment of post-RYGB bone mineral density loss and vitamin D deficiency as well as to reverse myeloid lineage commitment. HFD after RYGB continues to pose a significant multidimensional health risk.
Graphical Abstract








Similar content being viewed by others
Change history
18 January 2024
A Correction to this paper has been published: https://doi.org/10.1007/s11695-024-07064-0
References
Gasmi A, et al. Micronutrients deficiences in patients after bariatric surgery. Eur J Nutr. 2022;61(1):55–67.
Kushner R. Managing the obese patient after bariatric surgery: a case report of severe malnutrition and review of the literature. JPEN J Parenter Enteral Nutr. 2000;24(2):126–32.
Lim CH, et al. The future of the Roux-en-Y gastric bypass. Expert Rev Gastroenterol Hepatol. 2016;10(7):777–84.
Nance K, Acevedo MB, Pepino MY. Changes in taste function and ingestive behavior following bariatric surgery. Appetite. 2020;146:104423.
Mathes CM. Taste- and flavor-guided behaviors following Roux-en-Y gastric bypass in rodent models. Appetite. 2020;146:104422.
Jackness C, et al. Very low-calorie diet mimics the early beneficial effect of Roux-en-Y gastric bypass on insulin sensitivity and beta-cell function in type 2 diabetic patients. Diabetes. 2013;62(9):3027–32.
Yoshino M, et al. Effects of diet versus gastric bypass on metabolic function in diabetes. N Engl J Med. 2020;383(8):721–32.
Fischer IP, et al. A history of obesity leaves an inflammatory fingerprint in liver and adipose tissue. Int J Obes. 2018;42(3):507–17 (Lond).
Stevenson M, et al. Surgical mouse models of vertical sleeve gastrectomy and Roux-en Y gastric bypass: a review. Obes Surg. 2019;29(12):4084–94.
Tordoff MG, Ellis HT. Obesity in C57BL/6J mice fed diets differing in carbohydrate and fat but not energy content. Physiol Behav. 2022;243:113644.
Cottam MA, et al. Multiomics reveals persistence of obesity-associated immune cell phenotypes in adipose tissue during weight loss and weight regain in mice. Nat Commun. 2022;13(1):2950.
Christ A, et al. Western diet triggers NLRP3-dependent innate immune reprogramming. Cell. 2018;172(1–2):162-175 e14.
Weinstock A, Brown EJ, Garabedian ML, Pena S, Sharma M, Lafaille J, et al. Correction Weinstock A, et al. Single-Cell RNA Sequencing of Visceral Adipose Tissue Leukocytes Reveals that Caloric: Restriction Following Obesity Promotes the Accumulation of a Distinct Macrophage Population with Features of Phagocytic Cells. Immunometabolism. 2019;1:e190008. https://doi.org/10.20900/immunometab20190016.
Stevenson M, et al. RYGB is more effective than VSG at protecting mice from prolonged high-fat diet exposure: an occasion to roll up our sleeves? Obes Surg. 2021;31(7):3227–41.
Hao Z, et al. Development and verification of a mouse model for Roux-en-Y gastric bypass surgery with a small gastric pouch. PLoS ONE. 2013;8(1):e52922.
Kraus D, Yang Q, Kahn BB. Lipid extraction from mouse feces. Bio Protoc. 2015;5(1):e1375. https://doi.org/10.21769/bioprotoc.1375.
Gargiulo S, et al. Evaluation of growth patterns and body composition in C57Bl/6J mice using dual energy X-ray absorptiometry. Biomed Res Int. 2014;2014:253067.
Frost HM. Bone “mass” and the “mechanostat”: a proposal. Anat Rec. 1987;219(1):1–9.
Pearson OM, Lieberman DE. The aging of Wolff’s “law”: ontogeny and responses to mechanical loading in cortical bone. Am J Phys Anthropol. 2004;Suppl 39:63–99.
Shapses SA, Sukumar D. Bone metabolism in obesity and weight loss. Annu Rev Nutr. 2012;32:287–309.
Mechanick JI, et al. Clinical practice guidelines for the perioperative nutrition, metabolic, and nonsurgical support of patients undergoing bariatric procedures - 2019 update: cosponsored by American Association of Clinical Endocrinologists/American College of Endocrinology, The Obesity Society, American Society for Metabolic & Bariatric Surgery, Obesity Medicine Association, and American Society of Anesthesiologists. Surg Obes Relat Dis. 2020;16(2):175–247.
O’Kane M, et al. British Obesity and Metabolic Surgery Society Guidelines on perioperative and postoperative biochemical monitoring and micronutrient replacement for patients undergoing bariatric surgery-2020 update. Obes Rev. 2020;21(11):e13087.
Kondo M. Lymphoid and myeloid lineage commitment in multipotent hematopoietic progenitors. Immunol Rev. 2010;238(1):37–46.
van den Berg SM, et al. Diet-induced obesity in mice diminishes hematopoietic stem and progenitor cells in the bone marrow. FASEB J. 2016;30(5):1779–88.
Bowers E, Singer K. Obesity-induced inflammation: The impact of the hematopoietic stem cell niche. JCI Insight. 2021;6(3):e145295. https://doi.org/10.1172/jci.insight.145295
Xu Y, Murphy AJ, Fleetwood AJ. Hematopoietic progenitors and the bone marrow niche shape the inflammatory response and contribute to chronic disease. Int J Mol Sci. 2022;23(4).
Sandvik J, et al. Iron deficiency and anemia 10 years after Roux-en-Y gastric bypass for severe obesity. Front Endocrinol. 2021;12:679066 (Lausanne).
Muller TD, Klingenspor M, Tschop MH. Revisiting energy expenditure: how to correct mouse metabolic rate for body mass. Nat Metab. 2021;3(9):1134–6.
Virtue S, Lelliott CJ, Vidal-Puig A. What is the most appropriate covariate in ANCOVA when analysing metabolic rate? Nat Metab. 2021;3(12):1585.
Archer J. Rodent sex differences in emotional and related behavior. Behav Biol. 1975;14(4):451–79.
Kaiyala KJ, Schwartz MW. Toward a more complete (and less controversial) understanding of energy expenditure and its role in obesity pathogenesis. Diabetes. 2011;60(1):17–23.
Tschop MH, et al. A guide to analysis of mouse energy metabolism. Nat Methods. 2011;9(1):57–63.
Speakman JR. Measuring energy metabolism in the mouse - theoretical, practical, and analytical considerations. Front Physiol. 2013;4:34.
Fernandez-Verdejo R, et al. Progress and challenges in analyzing rodent energy expenditure. Nat Methods. 2019;16(9):797–9.
Buzby GP, et al. Prognostic nutritional index in gastrointestinal surgery. Am J Surg. 1980;139(1):160–7.
Keller U. Nutritional laboratory markers in malnutrition. J Clin Med. 2019;8(6):775. https://doi.org/10.3390/jcm8060775.
Hernandez-Martinez A, et al. Changes in volumetric bone mineral density and bone quality after Roux-en-Y gastric bypass: a meta-analysis with meta-regression. Obes Rev. 2022;23(8):e13479.
Paccou J, et al. Bariatric surgery and osteoporosis. Calcif Tissue Int. 2022;110(5):576–91.
Corbeels K, et al. The curious fate of bone following bariatric surgery: bone effects of sleeve gastrectomy (SG) and Roux-en-Y gastric bypass (RYGB) in mice. Int J Obes. 2020;44(10):2165–76 (Lond).
Yu EW, et al. Cortical and trabecular deterioration in mouse models of Roux-en-Y gastric bypass. Bone. 2016;85:23–8.
Zahedi B, et al. The PYY/Y2R-deficient male mouse is not protected from bone loss due to Roux-en-Y gastric bypass. Bone. 2023;167:116608.
Mangan A, et al. Iron and vitamin D/calcium deficiency after gastric bypass: mechanisms involved and strategies to improve oral supplement disposition. Curr Drug Metab. 2019;20(3):244–52.
Laird E, et al. Vitamin D and bone health: potential mechanisms. Nutrients. 2010;2(7):693–724.
Longo AB, Ward WE. PUFAs, Bone mineral density, and fragility fracture: findings from human studies. Adv Nutr. 2016;7(2):299–312.
Devlin MJ, et al. Differential effects of high fat diet and diet-induced obesity on skeletal acquisition in female C57BL/6J vs. FVB/NJ mice. Bone Rep. 2018;8:204–14.
Drincic AT, et al. Volumetric dilution, rather than sequestration best explains the low vitamin D status of obesity. Obesity. 2012;20(7):1444–8 (Silver Spring).
Park CY, et al. Effects of high fat diet-induced obesity on vitamin D metabolism and tissue distribution in vitamin D deficient or supplemented mice. Nutr Metab. 2020;17:44 (Lond).
Benova A, Tencerova M. Obesity-induced changes in bone marrow homeostasis. Front Endocrinol. 2020;11:294 (Lausanne).
Ambrosi TH, et al. Adipocyte accumulation in the bone marrow during obesity and aging impairs stem cell-based hematopoietic and bone regeneration. Cell Stem Cell. 2017;20(6):771-784 e6.
Chen Q, et al. Fate decision of mesenchymal stem cells: adipocytes or osteoblasts? Cell Death Differ. 2016;23(7):1128–39.
Singer K, et al. Diet-induced obesity promotes myelopoiesis in hematopoietic stem cells. Mol Metab. 2014;3(6):664–75.
Nagareddy PR, et al. Adipose tissue macrophages promote myelopoiesis and monocytosis in obesity. Cell Metab. 2014;19(5):821–35.
Griffin C, et al. TLR4, TRIF, and MyD88 are essential for myelopoiesis and CD11c(+) adipose tissue macrophage production in obese mice. J Biol Chem. 2018;293(23):8775–86.
Cortez M, et al. A high-fat diet increases IL-1, IL-6, and TNF-alpha production by increasing NF-kappaB and attenuating PPAR-gamma expression in bone marrow mesenchymal stem cells. Inflammation. 2013;36(2):379–86.
Reilly SM, Saltiel AR. Adapting to obesity with adipose tissue inflammation. Nat Rev Endocrinol. 2017;13(11):633–43.
van der Heijden RA, et al. High-fat diet induced obesity primes inflammation in adipose tissue prior to liver in C57BL/6j mice. Aging. 2015;7(4):256–68 (Albany NY).
Sayadi Shahraki M, et al. Bone health after bariatric surgery: consequences, prevention, and treatment. Adv Biomed Res. 2022;11:92.
Stein EM, Silverberg SJ. Bone loss after bariatric surgery: causes, consequences, and management. Lancet Diabetes Endocrinol. 2014;2(2):165–74.
Yao Y, et al. The macrophage-osteoclast axis in osteoimmunity and osteo-related diseases. Front Immunol. 2021;12:664871.
Samuel S, Sitrin MD. Vitamin D’s role in cell proliferation and differentiation. Nutr Rev. 2008;66(10 Suppl 2):S116–24.
Gesquiere I, et al. Iron deficiency after Roux-en-Y gastric bypass: insufficient iron absorption from oral iron supplements. Obes Surg. 2014;24(1):56–61.
Srivastava A, et al. Reversal of NAFLD after VSG is independent of weight-loss but RYGB offers more efficacy when maintained on a high-fat diet. Obes Surg. 2022;32(6):2010–22.
Barrientos T, et al. Metabolic catastrophe in mice lacking transferrin receptor in muscle. EBioMedicine. 2015;2(11):1705–17.
Ikeda Y, Funamoto M, Tsuchiya K. The role of iron in obesity and diabetes. J Med Invest. 2022;69(1.2):1–7.
Hilton C, et al. Iron, glucose and fat metabolism and obesity: an intertwined relationship. Int J Obes. 2023;47(7):554–63 (Lond).
Nazari M, et al. Iron chelation increases beige fat differentiation and metabolic activity, preventing and treating obesity. Sci Rep. 2022;12(1):776.
Hankir MK, Seyfried F. Do bariatric surgeries enhance brown/beige adipose tissue thermogenesis? Front Endocrinol. 2020;11:275 (Lausanne).
orrigan JK, Ramachandran D, He Y, Palmer CJ, Jurczak MJ, Chen R, et al. Mouse Metabolic Phenotyping Center Energy Balance Working Group; Banks AS. A big-data approach to understanding metabolic rate and response to obesity in laboratory mice. Elife. 2020;9:e53560. https://doi.org/10.7554/eLife.53560.
Kumar R, et al. Fat malabsorption and increased intestinal oxalate absorption are common after Roux-en-Y gastric bypass surgery. Surgery. 2011;149(5):654–61.
Mahawar KK, Sharples AJ. Contribution of malabsorption to weight loss after Roux-en-Y gastric bypass: a systematic review. Obes Surg. 2017;27(8):2194–206.
Cho YM. A gut feeling to cure diabetes: potential mechanisms of diabetes remission after bariatric surgery. Diabetes Metab J. 2014;38(6):406–15.
Vega GL, Grundy SM. Metabolic risk susceptibility in men is partially related to adiponectin/leptin ratio. J Obes. 2013;2013:409679.
Fruhbeck G, et al. Adiponectin-leptin ratio: a promising index to estimate adipose tissue dysfunction. Relation with obesity-associated cardiometabolic risk. Adipocyte. 2018;7(1):57–62.
Frühbeck G, Catalán V, Rodríguez A, Ramírez B, Becerril S, Salvador J, et al. Adiponectin-leptin ratio is a functional biomarker of adipose tissue inflammation. Nutrients. 2019;11(2):454. https://doi.org/10.3390/nu11020454.
Becerril S, Rodríguez A, Catalán V, Ramírez B, Mentxaka A, Neira G, et al. Sex- and age-dependent changes in the adiponectin/leptin ratio in experimental diet-induced obesity in mice. Nutrients. 2022;15(1):73. https://doi.org/10.3390/nu15010073.
Carswell KA, et al. A systematic review and meta-analysis of the effect of gastric bypass surgery on plasma lipid levels. Obes Surg. 2016;26(4):843–55.
Kaufman S, et al. Roux-en-Y gastric bypass surgery reprograms enterocyte triglyceride metabolism and postprandial secretion in rats. Mol Metab. 2019;23:51–9.
Lutz TA, Bueter M. Physiological mechanisms behind Roux-en-Y gastric bypass surgery. Dig Surg. 2014;31(1):13–24.
Fakhry TK, et al. Bariatric surgery improves nonalcoholic fatty liver disease: a contemporary systematic review and meta-analysis. Surg Obes Relat Dis. 2019;15(3):502–11.
Shalhub S, et al. The importance of routine liver biopsy in diagnosing nonalcoholic steatohepatitis in bariatric patients. Obes Surg. 2004;14(1):54–9.
Lefere S, et al. Bariatric surgery and the liver-mechanisms, benefits, and risks. Obes Rev. 2021;22(9):e13294.
Lee Y, et al. Complete resolution of nonalcoholic fatty liver disease after bariatric surgery: a systematic review and meta-analysis. Clin Gastroenterol Hepatol. 2019;17(6):1040-1060 e11.
Sandvik ECS, Aasarød KM, Johnsen G, Hoff DAL, Kulseng B, Hyldmo ÅA, et al. The effect of roux-en-y gastric bypass on non-alcoholic fatty liver disease fibrosis assessed by FIB-4 and NFS Scores—An 11.6-year follow-up study. J Clin Med. 2022;11:4910.
Głuszyńska P, Lemancewicz D, Dzięcioł JB, Razak Hady H. Non-alcoholic fatty liver disease (NAFLD) and bariatric/metabolic surgery as its treatment option: A review. J Clin Med. 2021;10(24):5721. https://doi.org/10.3390/jcm10245721.
Acknowledgements
We would like to thank the Comparative Medicine Division for their help and kind support throughout the duration of the study.
Funding
EAF and MN were supported by NIH grants HL131481 and HL084312.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethical Approval
All experiments and animal care protocols were approved by NYU Grossman Long Island School of Medicine’s Institutional Animal Use and Care Committee, which adheres to guidelines provided by the National Institutes of Health.
Informed Consent
Informed consent does not apply.
Conflict of Interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Key Points
• After RYGB, body weight, fat mass, and lean mass are similar regardless of diet.
• RYGB and HFD are independently detrimental to BMD and vitamin D levels.
• HFD promotes HSPC differentiation and myeloid skewing independent of RYGB surgery.
• HFD after RYGB impairs improvements to hyperglycemia, dyslipidemia, and NAFLD.
Supplementary Information
Below is the link to the electronic supplementary material.
Supplementary file1
(DOCX 4.83 MB)
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Stevenson, M., Srivastava, A., Nacher, M. et al. The Effect of Diet Composition on the Post-operative Outcomes of Roux-en-Y Gastric Bypass in Mice. OBES SURG 34, 911–927 (2024). https://doi.org/10.1007/s11695-023-07052-w
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11695-023-07052-w