Abstract
Purpose
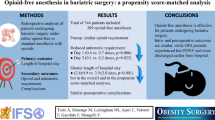
Opioid-free anesthesia (OFA) is an alternative to conventional opioid-based anesthesia (OBA) in patients undergoing bariatric surgery. Several small studies and a meta-analysis have suggested advantages of OFA for bariatric surgery, but current evidence is still contradictory, and a universally accepted concept has not yet been established. The purpose of this study was to determine whether patients undergoing bariatric surgery experience less postoperative pain and better postoperative recovery when anesthetized with an OFA regimen than with an OBA regimen.
Materials and Methods
This prospective observational cohort study, conducted between October 2020 and July 2021, compared patients receiving OFA with patients receiving OBA. Patients were visited 24 and 48 h after the surgical procedure and asked about their postoperative pain using the visual analogue scale (VAS). Additionally, the quality of recovery-40 questionnaire (QoR-40) and the postoperative opioid requirements were recorded.
Results
Ninety-nine patients were included and analyzed in this study (OFA: N = 50; OBA: N = 49). The OFA cohort exhibited less postoperative pain than the OBA cohort within 24 h (VAS median [interquartile range (IQR)]: 2.2 [1–4.4] vs. 4.1 [2–6.5]; P ≤ 0.001) and 48 h (VAS median [IQR]: 1.9 [0.4–4.1] vs. 3.1 [1.4–5.8]; P ≤ 0.001) postoperatively. Additionally, the OFA cohort had higher QoR-40 scores and required less opioid therapy postoperatively.
Conclusion
Based on our results the use of OFA for bariatric surgery results in less pain, reduced opioid requirements, and improved postoperative recovery—adding additional evidence regarding the use of OFA in everyday clinical practice.
Graphical Abstract

Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Opioid-free anesthesia (OFA) has been used for several years in an attempt to reduce risks and to improve recovery after surgery by decreasing opioid-induced side effects. For OFA, opioids are replaced by anesthetic adjuncts, such as ketamine, lidocaine, dexmedetomidine, and magnesium [1,2,3,4,5]. The use of OFA has been investigated for various types of surgery (e.g. gynecologic surgery [4, 6], bariatric surgery [1], cardiac surgery [7], urological surgery [8], orthopedic surgery [9]), and has been demonstrated to reduce postoperative pain [1, 10, 11] and postoperative nausea and vomiting (PONV) [6, 10, 11]. There is also evidence that OFA improves postoperative recovery [1, 4, 12]. However, with respect to adverse events, there is currently conflicting evidence regarding the safety of the use of OFA. A large prospective, randomized controlled trial had to be stopped early due to safety concerns due to the occurrence of bradycardia [13]. This contradicts two meta-analyses that assessed the evidence grade in this regard as moderate [14] or low [15].
Patients receiving bariatric surgery have a high risk for PONV [16, 17] and postoperative respiratory depression due to a high prevalence of obstructive sleep apnea syndrome in this patient population [18,19,20], necessitating an investigation of opiate-free anesthesia in this patient cohort [21]. In a recent meta-analysis of several small, randomized trials, Hung et al. demonstrated that the use of OFA in patients undergoing bariatric surgery resulted in a reduction in postoperative pain and PONV but not a reduction in postoperative opioid consumption within 24 h postoperatively [10]. This raises the question of whether these findings can be confirmed in a real-world scenario that is, in everyday clinical practice without a controlled trial setting. Furthermore, only a period of 24 h postoperatively was evaluated; thus, potential differences in postoperative pain over a longer postoperative period remain unclear. No significant difference was found for postoperative recovery (measured by the quality of recovery-40 questionnaire [QoR-40]) [22], but only two studies could be used to analyze this parameter, and in one of them, an additional bilateral oblique subcostal transverse abdominis plane block was performed in both groups [23], which may distort comparability. In a large retrospective analysis, the use of OFA was shown to result in a shorter LOS, indicating better recovery [24].
At our center, an OFA regimen has been used in parallel with an opioid-based regime (OBA) in bariatric surgery since June 2018. This study aimed to investigate the effects of these concepts on postoperative pain and recovery after a period of 48 h in patients who underwent bariatric surgery in everyday clinical practice.
Materials and Methods
Study Design
This prospective single-center cohort study examined postoperative pain, perioperative opioid consumption, PONV and the quality of recovery in patients undergoing first-intervention bariatric surgery between October 2020 and July 2021 at the Medical University of Vienna, Austria. We compared patients receiving OFA with patients receiving OBA. Informed consent was obtained from all individual participants included in the study. The study was approved by the Ethics Committee of the Medical University of Vienna (EK 1748/2020) and was performed in accordance with the principles of Good Clinical Practice and the Declaration of Helsinki.
Inclusion Criteria
The operating room schedule was regularly screened for eligible study participants, and eligible patients were contacted. Patients with a body mass index (BMI) greater than 35 kg/m2 undergoing first-time bariatric surgery under general anesthesia were included. Patient age was restricted to 18–65 years, and written informed consent was required before inclusion. Only patients who could be interviewed postoperatively were included. If a postoperative survey was not possible (e.g. study team was unavailable or language barrier), the patient was not included. Patients for whom the anesthetic regimen was changed intraoperatively (from OFA to OBA or vice versa) were excluded. When the sample size was reached in one cohort, no more patients were included in this cohort.
Cohorts
Patients were assigned to respective cohorts according to the type of anesthesia (OFA/OBA) performed.
Anesthetic Regimens Used/Treatment
The form of anesthesia (OFA/OBA) used was chosen by the anesthetist in charge and was determined on the basis of the standard operating procedure (SOP) of the respective operating room of the respective area. In our center, there are two sections of operating rooms performing bariatric surgery. In section 1, mainly OFA is applied, in section 2 OBA. Whether a patient is assigned to section 1 or 2 is random. To provide data from everyday clinical practice without a controlled trial setting, the study team had no influence on the treatment overall or choice of anesthesia at any time. OFA was performed according to an SOP. For anesthesia induction, a continuous intravenous infusion of s-ketamine (1.25 mg/ml), dexmedetomidine (10 µg/ml), and lidocaine (10 mg/ml) was started at 20 ml/h (s-ketamine: 25 mg/h; dexmedetomidine: 200 µg/h; lidocaine: 200 mg/h). Then, 200–250 mg propofol and 100 mg rocuronium were administered.
For maintenance, depending on the individual requirements of the patient, the syringe pump was set at 5 to 10 ml/h (s-ketamine: 6.25–12.5 mg/h; dexmedetomidine 50–100 µg/h; lidocaine 50–100 mg/h), and a volatile anesthetic (sevoflurane/desflurane) was administered. In addition, metamizole (2.5 g), magnesium sulfate (2–4 g), and parecoxib (40 mg) or diclofenac (75 mg) were administered intraoperatively. In the recovery room, continuous infusion could be continued at 5 ml/h as first-line analgesic therapy. Piritramide was available as a rescue therapy. This SOP is a modification of Mulier’s OFA protocol [1].
OBA was conducted by administering fentanyl, propofol, and rocuronium for induction and remifentanil in combination with a volatile anesthetic (sevoflurane/desflurane) for maintenance. Piritramide was the first line of analgesic therapy for the treatment of acute postoperative pain.
Detailed descriptions of the anesthetic concepts are provided in the supplementary material.
Determination of Outcomes
Participants were questioned 24 h and 48 h after surgery to assess pain perception (using the visual analogue scale (VAS)) at different time points and postoperative recovery (using the quality of recovery-40 (QoR-40) questionnaire) during the first 48 h after surgery. In addition, the opioid (piritramide) requirement was recorded 24 h after surgery, based on documentation in the patient files.
Primary Outcome
The primary endpoint of this study was the difference in the VAS within the first 24 h after surgery between the two cohorts. For this purpose, the participating patients were asked about their pain perception five times within this period: immediately after surgery, during initial mobilization, in the evening after surgery, in the morning on the first postoperative day (POD) day, and at noon on the first POD.
Secondary Outcomes
For postoperative pain assessment between 24 and 48 h, patients were asked about their pain perception at three additional time points using the VAS score: in the evening on the first POD, in the morning on the second POD, and at noon on the second POD. Postoperative recovery was determined using the QoR-40 questionnaire 24 and 48 h postoperatively. To assess PONV separately, we evaluated the three PONV-specific parameters (nausea, vomiting, dry retching) of the QoR-40 questionnaire at 24 h (PONV-score: 3 = worst, 15 = best) and recorded whether droperidol was administered in the post anesthesia care unit (PACU). Postoperative opioid consumption within 24 h was recorded in the recovery room and in the ward in both cohorts, as well as in the administration of s-ketamine, dexmedetomidine, and lidocaine in the OFA cohort in the recovery room. The length of hospital stay was documented as well as whether patients were discharged within 24 to 48 h postoperatively before the second survey.
Statistical Analysis
No formal sample size calculation was performed. We planned to observe 50 patients in the OFA group and 50 patients in the OBA group. The number of cases was determined to allow for examining a larger patient collective than had previously been done in smaller randomized studies [2,3,4,5,6]. Normal distribution for continuous data was tested using the Kolmogorov–Smirnov test. Normally distributed data are presented as mean (SD), and non-normally distributed data are presented as median (interquartile range [IQR], 25th to 75th percentile). The main endpoint of this study (VAS within 24 h) was compared between the two groups with a Mann–Whitney U test. For the secondary outcome parameters, exploratory Mann–Whitney U-tests, exploratory t-tests, and an exploratory chi-square test were performed (depending on the normal distribution); accordingly, no strategy for multiple testing was used. We considered two-sided P values ≤ 0.05 to be statistically significant. Differences in medians are reported using the Hodges–Lehmann estimator.
All statistical tests were performed using SPSS Statistics (IBM SPSS Statistics for Windows, Version 27.0, IBM Corp.), and all graphic representations were performed using GraphPad Prism (GraphPad Prism for Windows, Version 9.4.1. GraphPad Software). There was no need to follow up with the patients.
Results
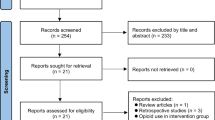
During the investigation period, 171 patients were screened, 99 of whom were included and analyzed in this study: 50 patients in the OFA cohort and 49 patients in the OBA cohort. The flow chart is presented in Fig. 1. One patient in the OBA cohort was incorrectly included due to a transcription error and was therefore excluded from the data analysis. The investigation period was from October 2020 to July 2021. The morphometric and baseline parameters of the study participants are presented in Table 1.
Primary Outcome
The median [IQR] VAS in the first 24 h postoperatively was 2.2 [1–4.4] in the OFA cohort and 4.1 [2–6.5] in the OBA cohort (Hodges–Lehmann estimator of the difference, -1.5; 95.0% CI, -2 to -1; Mann–Whitney U-Test P ≤ 0.001). The course of postoperative pain in both cohorts over 48 h is presented in Fig. 2. Figure 3 shows the postoperative pain within 24 h for the different types of surgery.
Secondary Outcomes
Numeric secondary outcome parameters are shown in Table 2. A total of 26 (52%) patients in the OFA cohort received continuous infusion of s-ketamine, dexmedetomidine, and lidocaine in the recovery room, whereas 15 (30%) patients received neither piritramide nor a continuous infusion of s-ketamine, dexmedetomidine, and lidocaine. We observed that 9 (18%) patients in the OBA cohort did not require piritramide. Postoperative opioid consumption (Piritramide) was lower in the OFA group (OFA: 0 [0–3.4], OBA: 6[3–9]; P ≤ 0,001). 26 (52%) of 50 patients in the OFA group and 38 (77.6%) of 49 patients in the OBA group received droperidol in the PACU (chi-square test, P = 0.008).
We found statistically significant differences regarding QoR-40 scores after 24 h (OFA: 166 (13.5), OBA: 155 (16.5); P ≤ 0.001) and 48 h (OFA: 183 (9.7), OBA: 173 (11.6); ≤ 0.001) in favor of the OFA group. The differences in QoR-40 regarding postoperative pain are shown in Table 3. The median LOS was 4 days [3–4] and 4 days [3–4] in the OFA and OBA groups, respectively (Mann–Whitney U Test, P = 0,609). In the OFA cohort, 15 (30%) patients were discharged between 24 and 48 h after surgery, and 12 (24%) patients were discharged between 24 and 48 h in the OBA cohort (chi-square test, P = 0.538).
Discussion
The use of opiate-free anesthesia is reported to be associated with less postoperative pain and better postoperative recovery in bariatric surgery, but there is no universally accepted concept yet, and some aspects have not been adequately investigated. In this prospective cohort study comparing an OFA regimen with an OBA regimen in patients undergoing bariatric surgery, patients were surveyed 24 and 48 h postoperatively regarding their postoperative pain, as well as their postoperative recovery, to investigate whether the results found in a controlled trial setting can be confirmed in everyday clinical practice. Our hypothesis was that the results found in controlled trials, could be transferred to everyday clinical practice without a controlled trial setting and that, accordingly, patients receiving OFA would have less postoperative pain and better postoperative recovery. We observed statistically significant differences in postoperative pain and postoperative recovery between the cohorts over a period of 48 h postoperatively. Patients in the OFA cohort had significantly lower pain scores, lower opioid requirements, and recovered faster than patients in the OBA cohort.
Given that patients undergoing bariatric surgery have high rates of PONV [16, 17] and a higher risk of postoperative respiratory depression and sleep apnea [18, 25], the investigation of OFA for bariatric surgery is scientifically and clinically relevant. Despite ongoing controversy regarding the beneficial effects of OFA concepts [13], our results are comparable to those of smaller randomized controlled studies [1,2,3, 8] and previous results [10]. Our study not only confirms these results but also demonstrates that the beneficial effects of OFA for bariatric surgery can be achieved in everyday clinical practice and not only in a controlled trial setting. Our results are also in line with the findings of Urvoy et al., who conducted a partly similar observational study but included patients receiving total hip arthroplasty [9].
We could show a statistically significant difference regarding PONV within 24 h postoperatively in favor of the OFA group. These findings are in line with the results of previous investigations [10, 14] and confirm that the application of OFA leads to a reduction of PONV in patients receiving bariatric surgery in everyday clinical practice. The relatively large difference in postoperative opioid requirements observed in our study might be partly due to the intraoperative s-ketamine, dexmedetomidine, and lidocaine infusion being continued in the recovery room. Consequently, opioid administration was the second choice for treating acute postoperative pain in the OFA group. Therefore, whether strict intraoperative use of OFA (without continuing into the postoperative period) would have led to a similar reduction in postoperative opioid administration remains unclear.
We assumed, from clinical experience and due to the fact that these parameters are included in the QoR-40, that the differences found in the QoR-40 were related to the outcome parameters of postoperative pain, PONV, and postoperative opioid consumption. This would also correspond to the findings presented in Table 3. Accordingly, we can conclude that the application of OFA leads to an improved patient experience due to the improvement of several parameters that influence postoperative recovery.
According to a retrospective study by Mulier et al., OFA improves the outcome of bariatric procedures; thus, this regimen can be assumed to be safe for use in this patient population [24]. Our study was not designed to specifically investigate the safety of OFA (e.g. the occurrence of bradycardia, as described by Beloeil et al. in 2021 [15]). We are therefore unable to draw definite conclusions regarding the safety of OFA, but we are currently performing a retrospective evaluation of this issue at our center.
We found no difference regarding LOS, even though the patients in the OFA group showed statistically significant differences in the QoR-40 questionnaire 24 and 48 h postoperatively. These findings contradict the results of a comprehensive retrospective analysis of a large patient collective done by Mulier et al. [24], which showed that patients receiving OFA had a shorter LOS, but are in line with the findings of a recent meta-analysis, even though only two studies were included for this parameter in the analysis [10]. However, it must be taken into account that LOS is not solely dependent on the anesthesiologic procedure and can be influenced by a variety of other factors (e.g. different health care systems, center-specific SOPs). Compared with other studies, our patients had a longer LOS (4 days vs. 3 days) [24, 26]. We suspect that this difference may be due to the fact that in our center, patients are admitted the day before surgery.
A limitation of this study is that it was conducted at a single center, limiting the generalizability of our results. Due to the size of our department (approximately 180 anesthetists), we nevertheless believe that these methods can be applied by a large variety of anesthetists.
Conclusion
The results of this study showed that the introduction of a concept for OFA in clinical routine practice was associated with less postoperative pain, lower opioid requirements and less PONV and patients demonstrated improved postoperative recovery. Further studies are needed to determine the best anesthetic protocol and to investigate safety aspects with this form of anesthesia.
Data Availability
The data will be made available on justified request to the corresponding author.
References
Mulier JP, Wouters R, Dillemans B, et al. A randomized controlled, double-blind trial evaluating the effect of opioid-free versus opioid general anaesthesia on postoperative pain and discomfort measured by the QoR-40. J Clin Anesth Pain Med. 2018;6:2.
Mansour MA, Mahmoud AAA, Geddawy M. Nonopioid versus opioid based general anesthesia technique for bariatric surgery: a randomized double-blind study. Saudi J Anaesth. 2013;7:387–91.
Bakan M, Umutoglu T, Topuz U, et al. Opioid-free total intravenous anesthesia with propofol, dexmedetomidine and lidocaine infusions for laparoscopic cholecystectomy: a prospective, randomized, double-blinded study. Braz J Anesthesiol Elsevier. 2015;65:191–9.
Hakim KYK, Wahba WZB. Opioid-free total intravenous anesthesia improves postoperative quality of recovery after ambulatory gynecologic laparoscopy. Anesth Essays Res. 2019;13:199–203.
Tochie JN, BengonoBengono RS, Metogo JM, et al. The efficacy and safety of an adapted opioid-free anesthesia regimen versus conventional general anesthesia in gynecological surgery for low-resource settings: a randomized pilot study. BMC Anesthesiol. 2022;22:325.
Chen L, He W, Liu X, et al. Application of opioid-free general anesthesia for gynecological laparoscopic surgery under ERAS protocol: a non-inferiority randomized controlled trial. BMC Anesthesiol. 2023;23:34.
Aguerreche C, Cadier G, Beurton A, et al. Feasibility and postoperative opioid sparing effect of an opioid-free anaesthesia in adult cardiac surgery: a retrospective study. BMC Anesthesiol. 2021;21:166.
Bhardwaj S, Garg K, Devgan S. Comparison of opioid-based and opioid-free TIVA for laparoscopic urological procedures in obese patients. J Anaesthesiol Clin Pharmacol. 2019;35:481–6.
Urvoy B, Aveline C, Belot N, et al. Opioid-free anaesthesia for anterior total hip replacement under general anaesthesia: the Observational Prospective Study of Opiate-free Anesthesia for Anterior Total Hip Replacement trial. Br J Anaesth. 2021;126:e136–9.
Hung K-C, Chiu C-C, Hsu C-W, et al. Impact of opioid-free anesthesia on analgesia and recovery following bariatric surgery: a meta-analysis of randomized controlled studies. Obes Surg. 2022;32:3113–24.
Mulier H, De Frene B, Benmeridja L, et al. Impact of opioid-free anesthesia on complications after deep inferior epigastric perforator flap surgery: a retrospective cohort study. J Plast Reconstr Aesthetic Surg JPRAS. 2021;74:504–11.
Choi H, Song JY, Oh EJ, et al. The effect of opioid-free anesthesia on the quality of recovery after gynecological laparoscopy: a prospective randomized controlled trial. J Pain Res. 2022;15:2197–209.
Beloeil H, Garot M, Lebuffe G, et al. Balanced opioid-free anesthesia with dexmedetomidine versus balanced anesthesia with remifentanil for major or intermediate noncardiac surgery. Anesthesiology. 2021;134:541–51.
Olausson A, Svensson CJ, Andréll P, Jildenstål P, Thörn S-E, Wolf A. Total opioid-free general anaesthesia can improve postoperative outcomes after surgery, without evidence of adverse effects on patient safety and pain management: A systematic review and meta-analysis. Acta Anaesthesiol Scand. 2022;66:170–85.
Salomé A, Harkouk H, Fletcher D, et al. Opioid-free anesthesia benefit-risk balance: a systematic review and meta-analysis of randomized controlled trials. J Clin Med. 2021;10:2069.
Groene P, Eisenlohr J, Zeuzem C, et al. Postoperative nausea and vomiting in bariatric surgery in comparison to non-bariatric gastric surgery. Wideochirurgia Inne Tech Maloinwazyjne Videosurgery Miniinvasive Tech. 2019;14:90–5.
Halliday TA, Sundqvist J, Hultin M, et al. Post-operative nausea and vomiting in bariatric surgery patients: an observational study. Acta Anaesthesiol Scand. 2017;61:471–9.
Lopez PP, Stefan B, Schulman CI, et al. Prevalence of sleep apnea in morbidly obese patients who presented for weight loss surgery evaluation: more evidence for routine screening for obstructive sleep apnea before weight loss surgery. Am Surg. 2008;74:834–8.
Kositanurit W, Muntham D, Udomsawaengsup S, et al. Prevalence and associated factors of obstructive sleep apnea in morbidly obese patients undergoing bariatric surgery. Sleep Breath Schlaf Atm. 2018;22:251–6.
Gupta K, Nagappa M, Prasad A, et al. Risk factors for opioid-induced respiratory depression in surgical patients: a systematic review and meta-analyses. BMJ Open. 2018;8: e024086.
Feld JM, Laurito CE, Beckerman M, et al. Non-opioid analgesia improves pain relief and decreases sedation after gastric bypass surgery. Can J Anaesth J Can Anesth. 2003;50:336–41.
Myles PS, Weitkamp B, Jones K, et al. Validity and reliability of a postoperative quality of recovery score: the QoR-40. Br J Anaesth. 2000;84:11–5.
Ibrahim M, Elnabtity AM, Hegab A, et al. Combined opioid free and loco-regional anaesthesia enhances the quality of recovery in sleeve gastrectomy done under ERAS protocol: a randomized controlled trial. BMC Anesthesiol. 2022;22:29.
Mulier JP, Dillemans B. Anaesthetic factors affecting outcome after bariatric surgery, a retrospective levelled regression analysis. Obes Surg. 2019;29:1841–50.
de Raaff CAL, Gorter-Stam MAW, de Vries N, et al. Perioperative management of obstructive sleep apnea in bariatric surgery: a consensus guideline. Surg Obes Relat Dis. 2017;13:1095–109.
Berlier J, Carabalona J-F, Tête H, et al. Effects of opioid-free anesthesia on postoperative morphine consumption after bariatric surgery. J Clin Anesth. 2022;81: 110906.
Funding
Open access funding provided by Medical University of Vienna.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethical Approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed Consent
Informed consent was obtained from all individual participants included in the study.
Conflict of Interest
Stefan Ulbing: no conflicts of interest; Lukas Infanger: no conflict of interest; Edith Fleischmann: no conflict of interest; Gerhard Prager: no conflict of interest; Thomas Hamp: no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Key Points
• OFA was associated with less postoperative pain in clinical practice.
• OFA was associated with reduced postoperative opioid requirements.
• OFA was associated with improved postoperative recovery in clinical practice.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Ulbing, S., Infanger, L., Fleischmann, E. et al. The Performance of Opioid-Free Anesthesia for Bariatric Surgery in Clinical Practice. OBES SURG 33, 1687–1693 (2023). https://doi.org/10.1007/s11695-023-06584-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11695-023-06584-5