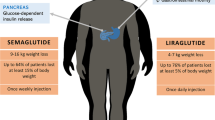
Abstract
Purpose
Weight regain after bariatric surgery occurs in up to a third of patients and reduces treatment-associated health benefits. The efficacy of glucagon-like peptide-1 receptor agonists (GLP1-RA) for treatment of type 2 diabetes mellitus and obesity is well established, but their role in the treatment of weight regain after bariatric surgery remains to be defined.
Materials and Methods
This was a single centre retrospective observational study conducted at a Swiss bariatric reference centre. Patients with 6 months of treatment with GLP1-RA, up until November 2021, due to weight regain after bariatric surgery were identified. Data on body weight and relevant clinical parameters were collected before and after 6 months of treatment with GLP1-RA. Data are presented as median (interquartile range).
Results
Fifty patients (82% female) were included. Before GLP1-RA treatment (liraglutide, n=29; semaglutide, n=21), weight and BMI were 90.5 kg (83.4, 107.9) and 34.0 kg/m2 (31.7, 38.7), respectively, with a post-bariatric weight regain of 15.1% (10.6, 22.8) of total body weight and 4.6 kg/m2 (3.3, 6.2). After 6 months of GLP1-RA treatment, a reduction in weight and BMI of 8.8% (5.2, 11.4) of total body weight and 2.9 kg/m2 (1.8, 4.0) was observed (P value <0.0001), corresponding to 67.4% (40.4, 92.2) of the weight regain. No serious adverse events were reported.
Conclusion
For patients experiencing weight regain after bariatric surgery, two-thirds of the weight regain can be safely lost with GLP1-RA, providing clinicians with a therapeutic option for this clinical challenge, and highlights the need for a large-scale randomized clinical trial.
Graphical Abstract

Similar content being viewed by others
Introduction
Bariatric surgery is currently the most efficient treatment for severe obesity, resulting in durable weight loss, improvement of cardiovascular risk factors and obesity-related comorbidities, and reduced all-cause mortality [1]. However, depending on the definition used, between one to two in five patients (16–37%) experience significant weight regain, thus reducing the long-term benefits of the procedure [2, 3]. An effective standard therapy for weight regain after bariatric surgery has not been established. Lifestyle measures are generally reinforced with additional behavioural intervention and occasionally pharmacotherapy. Revisional surgery might be considered appropriate when the underlying reason for weight regain is thought to be anatomical. Other procedures such as distal Roux-en-Y gastric bypass and biliopancreatic diversion are mostly reserved for refractory cases [4, 5].
Glucagon-like peptide-1 receptor agonists (GLP1-RAs) are the currently most efficacious weight lowering drugs. The efficacy and safety profile of the GLP1-RAs liraglutide and semaglutide are well-documented, with an average body weight loss of up to 15%, usually transient mild to moderate gastrointestinal side effects, and cardiorenal benefits [6,7,8]. Their role in the treatment of weight regain after bariatric surgery remains to be defined.
Reports on the efficacy of GLP1-RAs in patients with weight regain after bariatric surgery are mostly limited to liraglutide in observational studies or case reports [9,10,11,12,13,14,15,16,17,18,19,20]. The GRAVITAS trial randomized patients with persistent or recurrent type 2 diabetes (T2D) following bariatric surgery to receive liraglutide (1.8 mg, daily subcutaneous injection) or placebo. Although the primary outcome was change in HbA1c, the results showed a difference in mean weight loss of 4.2 kg in the liraglutide vs placebo group after 26 weeks of treatment [21]. Preliminary results of a randomized study of liraglutide (3.0 mg, daily subcutaneous injection) versus placebo for 12 months in patients with weight regain after Roux-en-Y gastric bypass show that 67.2% of the patients in the liraglutide group versus 4.4% in the placebo group lost at least 5% of their baseline body weight [22].
A recent review by Redmond et al. considered semaglutide a reasonable therapeutic option for weight regain after bariatric surgery [23], and the first data on the efficacy of semaglutide in this patient population was recently reported in a retrospective observational study from Germany, in patients with insufficient weight loss or weight regain after bariatric surgery. After 6 months of weekly subcutaneous therapy with semaglutide at a maximum dose of 0.5 mg, an average total body weight loss of 10.3% was observed [24].
Clinical studies have shown that the efficacy of weight loss with semaglutide is significantly better than with liraglutide [25, 26]. The latest published results comparing weight loss with weekly subcutaneous semaglutide (2.4 mg) vs daily subcutaneous liraglutide (3.0 mg) in patients with overweight or obesity without T2D demonstrated a total body weight loss of 15.8% with semaglutide vs 6.4% with liraglutide after 68 weeks of therapy [27].
In Switzerland, both liraglutide and semaglutide are reimbursed for the treatment of T2D and liraglutide in addition for the treatment of obesity. However, neither of the compounds is reimbursed in patients with previous bariatric surgery without T2D, mainly due to the lack of supportive clinical trial data. The price of liraglutide at the maximal approved daily subcutaneous dose of 1.8 mg for T2D and 3.0 mg for weight reduction (approximately 191 and 211 USD/month, respectively) is considerably higher than the cost of semaglutide (just below 140 USD/month at maximal weekly subcutaneous [1.0 mg] or daily oral [14 mg] dose). The price of semaglutide for weight reduction, if approved in Switzerland, is unknown. As an example, the cost for liraglutide 3.0 mg, daily subcutaneous injection, and semaglutide 2.4 mg, weekly subcutaneous injection, is in the United States comparable (approximately 1350 USD/month).
Therefore, to meet the needs of the patients attending our obesity outpatient clinic, GLP1-RAs are used both on- and off-label to treat weight regain after bariatric surgery. As semaglutide has so far not been approved for weight management in Switzerland, the dose currently approved for the treatment of T2D (1.0 mg, weekly subcutaneous injection) is prescribed to patients for whom treatment with liraglutide is not reimbursed or affordable.
In this retrospective observational study, we present the results of 6 months of treatment with the GLP1-RAs liraglutide and semaglutide in patients with weight regain after bariatric surgery. The aim is to document the efficacy and safety of prescribing both GLP1-RAs for weight regain after bariatric surgery in a real-world patient setting.
Patients and Methods
We performed a single centre retrospective observational study in the obesity outpatient reference centre at the Cantonal Hospital of St. Gallen, Switzerland. The centre provides medical care within the framework of the obligatory healthcare insurance, and the patients are therefore to be considered representative of the population of patients with obesity having undergone bariatric surgery in Switzerland. Patients with weight regain after bariatric surgery that were prescribed GLP1-RA and with 6 months of treatment available, up until November 2021, were identified. A written informed general consent was present for all patients included in the study. The study was approved by the regional ethics committee.
GLP1-RA for weight management was either prescribed on-label (daily subcutaneous injection of 3.0 mg liraglutide) or off-label with either semaglutide or liraglutide (the latter administered as daily subcutaneous injection of 1.8 mg before the higher 3.0 mg dose was available for weight management as of December 2016). As semaglutide was not approved for weight management in Switzerland, a dose approved for T2D was prescribed (1.0 mg, weekly subcutaneous injection or 14.0 mg, daily oral intake). Patient data was retrospectively collected from the clinical records. In general, patients were seen by the treating physician every three months after GLP1-RA therapy initiation. For various reasons, including the COVID-19 pandemic, this was not always possible, in which case data from the closest visit (6 months ± 1 month) was used. Weight regain was defined as any weight gain following the weight nadir at least 12 months after bariatric surgery. Patients with one or two prior bariatric operations were included. The indication to initiate GLP1-RA therapy and the agent prescribed was at the discretion of the treating physician, taking the overall weight status, cardiovascular risk profile, and patient preferences into consideration.
In general, patients were offered treatment with GLP1-RAs if weight regain occurred despite intensified lifestyle and behavioural intervention. Patients were made aware that treatment, if not reimbursed by the health insurance company, would be at their own expense. A stopping rule after 16 weeks of therapy was implemented in case treatment with liraglutide was reimbursed, requiring a minimum of 5% or 7% total body weight loss in patients with a BMI between 28 and 35 kg/m2 or ≥35 kg/m2, respectively, according to Swiss reimbursement rules based on post hoc analyses from the SCALE trials [28]. In case liraglutide therapy was self-paid by the patient, the stopping rule was used at the discretion of the treating physician. No stopping rule was used for semaglutide therapy. If clinically warranted, a potential anatomical cause for the weight regain (pouch-/stoma-dilation, gastro-gastric-fistula, or gastric sleeve dilation) was investigated with a barium meal test and/or an upper endoscopy. If an anatomical cause were identified, the choice between medical and surgical therapy would be thoroughly discussed with the patient. All patients undergoing bariatric surgery were regularly counselled by an in-house registered dietician both pre- and post-operatively, to the extent clinically warranted within the framework of the given cost reimbursement. In the event of weight regain, dietary counselling was again recommended.
The primary outcomes were change in total body weight and BMI and the percentage of weight regain lost following 6 months of GLP1-RA therapy.
The secondary end point was safety, based on any therapy-related adverse events reported in the patient records by the treating physician, including severity. Sensitivity analyses were performed to assess the influence of GLP1-RA agent (liraglutide vs semaglutide), time between bariatric surgery and GLP1-RA initiation below and above median value, diagnosis of T2D, sex, age below and above the median value, the presence of a potential anatomical cause for weight regain, weight regain below and above the median value, and self-payer status (among patients with liraglutide therapy), and outlier therapies (defined as any GLP1-RA application format prescribed to one patient only). Data are presented as median with interquartile range in brackets, if not otherwise indicated. Paired Student’s t tests were used to compare differences before and after 6 months of GLP1-RA treatment. Student’s t tests or Wilcoxon rank sum tests, as appropriate, were used to compare differences between groups in stratified analysis. A P value <0.05 was considered statistically significant.
Results
In total, 108 patients were identified that were prescribed GLP1-RA therapy due to weight regain after bariatric surgery up until the end of data collection in November 2021. The first patient visit for GLP1-RA initiation (baseline) took place in November 2016, and the last 6 months follow-up visit took place in November 2021. Of the 108 patients identified, 11 had not provided informed general consent, and 11 patients ended up never initiating treatment. Of the remaining 86 patients, 36 did not have 6 months follow-up data available leaving 50 patients (82% female) available for analysis (Fig. 1). Regarding type of surgery; 82.0% (n=41) had undergone proximal Roux-en-Y gastric bypass, 10.0% (n=5) sleeve gastrectomy, and 8.0% (n=4) distal Roux-en-Y gastric bypass (common channel <100 cm). Fourteen percent (n=7) of the patients had undergone two bariatric procedures, with adjustable gastric banding as the initial procedure in four patients and sleeve gastrectomy in three patients. Of the 50 patients included in the analysis, 29 underwent 6 months of treatment with liraglutide (3.0 mg [n=28] and 1.8 mg [n=1], daily subcutaneous injection) and 21 underwent treatment with semaglutide (1.0 mg, weekly subcutaneous injection [n=20] and 14 mg, daily oral intake [n=1]). Thirty-seven (74%) of the patients were self-payers. Since semaglutide is not reimbursed for weight reduction, all patients treated with semaglutide were self-payers, whereas 16 (55.2%) of the 29 patients treated with liraglutide were self-payers. Ten (20%) of the patients included had an identified potential anatomical cause for weight regain at baseline, five with a pouch-dilation, and three with a stoma-dilation and two with a combined pouch- and stoma-dilation. Patient characteristics pre-bariatric surgery, at weight nadir, GLP1-RA initiation (baseline), and after 6 months of therapy are presented in Table 1.
At baseline, 72.0 months (43.8, 96.0) after the last bariatric surgery, patients had regained 15.1% (10.6, 22.8) of total body weight or 4.6 kg/m2 (3.3, 6.2). From a weight of 90.5 kg (83.4, 107.9) and BMI of 34.0 kg/m2 (31.7, 38.7) at baseline, patients had lost 8.8% (5.2, 11.4) of total body weight and 2.9 kg/m2 (1.8, 4.0) after 6 months of treatment (P value <0.0001), corresponding to 67.4% (40.4, 92.2) of the weight regained after the last bariatric procedure (Fig. 2). Agent-specific total body weight loss was 7.3% (3.1, 10.3) for patients with liraglutide (n=29) and 9.8% (8.2, 13.0) for patients with semaglutide (n=21, P value <0.05). Overall, a total body weight loss of ≥5% of baseline weight was achieved by 76.0% (n=38), ≥10% loss by 38.0% (n=19), and ≥15% loss by 12.0% of the patients (n=6). In patients treated with liraglutide (n=29), a total body weight loss of ≥5% of baseline weight was achieved by 69.0% (n=20), ≥10% loss by 31.0% (n=9), and ≥15% loss by 3.5% of the patients (n=1). In patients treated with semaglutide (n=21), a total body weight loss of ≥5% of baseline weight was achieved by 85.7% (n=18), ≥10% loss by 47.6% (n=10), and ≥15% loss by 23.8% of the patients (n=5) (Fig. 3). Adverse events were reported in 36.0% of the patients, all of which were transient, considered mild, and primarily related to the gastrointestinal system (Table 2).
Various sensitivity analyses were performed. According to these, treatment with semaglutide (1.0 mg, weekly subcutaneous injection [n=20] or 14 mg, daily oral intake [n=1]) as compared to liraglutide (daily subcutaneous injection with 3.0 mg [n=28] or 1.8 mg [n=1]) resulted in a significantly greater reduction in BMI (3.9 kg/m2 (2.9, 4.8) vs 2.5 kg/m2 (1.1, 3.3), P value <0.001, Fig. 4). A tendency towards an increased effect of GLP1-RAs on weight loss if treatment was initiated ≥72 months as compared to <72 months after bariatric surgery was observed, albeit not statistically significant (−3.1 kg/m2 (−2.5, −4.6) vs −2.6 kg/m2 (−1.1, −3.6), P value =0.12, Supplement). Excluding outlier therapies (liraglutide, 1.8 mg daily subcutaneous injection [n=1] and semaglutide, 14 mg daily oral intake [n=1]) did not significantly change the overall results on body weight after 6 months GLP1-RA therapy or the results of the comparison between semaglutide and liraglutide (Supplement). Further baseline characteristics, such as diagnosis of T2D (of which 22.2% were treated with insulin), sex, age below and above the median value, the presence of a potential anatomical cause for weight regain, weight regain (%) below and above the median value, and self-payer status of the patients with liraglutide therapy, did not significantly influence the observed efficacy of the GLP1-RAs in reducing body weight (all P values >0.05, Supplement).
Conclusion
We performed a single centre retrospective observational study on the efficacy of the GLP1-RAs liraglutide and semaglutide in reducing weight in 50 patients with weight regain after bariatric surgery. The median percentage of total body weight loss following 6 months of GLP1-RA therapy was 8.8%. More than three in four patients lost more than 5% of their baseline weight, and more than one in three patients lost more than 10%. The median patient had lost 67.4% of the weight regained after the last bariatric procedure. Adverse events were documented for approximately a third of the patients, all of which were mild, transient, and primarily related to the gastrointestinal system, with no severe adverse events reported. Overall, our findings support the safe use of both GLP1-RAs to achieve a clinically significant weight loss of approximately two-thirds of the weight regained after bariatric surgery.
The magnitude of weight loss observed with GLP1-RA in this real-life patient population is consistent with findings from the SCALE, SUSTAIN, and STEP clinical trials, which are based on patients without bariatric surgery. In the SCALE obesity and prediabetes study, in patients with obesity without T2D, patients on liraglutide (3.0 mg, daily subcutaneous injection) lost 8.0% of their total body weight versus 2.6% in the placebo group after 56 weeks of treatment [29]. In the SCALE diabetes study, a total body weight loss of 6.0% was seen in patients with T2D randomized to liraglutide (3.0 mg, daily subcutaneous injection) versus 2.0% with placebo after 56 weeks of treatment [30]. In SUSTAIN 1 comparing semaglutide (0.5 or 1.0 mg, weekly subcutaneously injection) with placebo in patients with T2D and a mean BMI of 32.9 kg/m2, a total body weight loss of 4.5 kg (4.9%) and 1.0 kg (1.1%) was seen in the patients with semaglutide vs placebo after 30 weeks of treatment [31]. The STEP 1 and 2 studies, investigating the weight effect of semaglutide 2.4 mg weekly versus placebo over 68 weeks, reported a weight loss of 12.4% and 6.2%, respectively [32, 33]. Both in the SCALE and STEP studies, a weight plateau was usually seen 6–12 months after baseline.
Our results are also in line with non-randomized studies in patients with previous bariatric surgery. A prospective observational study from the United Arab Emirates including patients with previous bariatric surgery and obesity without T2D showed a 6.1% weight loss after 16 weeks of therapy with liraglutide (3.0 mg, daily subcutaneous injection) [15]. Similarly, an observational study from Canada demonstrated a 5.5% weight loss with liraglutide (3.0 mg, daily subcutaneous injection) over 7.6 months in patients with previous bariatric surgery [14]. In the retrospective study of Lautenbach et al., a mean weight loss of 10.3% with 85% of patients achieving a weight loss of ≥5% after 6 months GLP1-RA therapy was reported with semaglutide, 0.5 mg, weekly subcutaneous injection. In our study, the total body weight loss after 6 months of semaglutide, 1.0 mg, weekly subcutaneous injection, was 9.8% with 85.7% of the patients achieving a weight loss of ≥5%. Lautenbach et al. included patients without T2D with both insufficient weight loss and weight regain after bariatric surgery, and this difference in patient characteristics could possibly explain the similar weight loss obtained in both studies despite the difference in dose. The current study includes patients with T2D that have been shown to lose less weight with GLP1-RA therapy [29, 30, 32, 33], whereas the study by Lautenbach includes “poor” responders to bariatric surgery, which have been shown to have lower endogenous GLP-1 levels, thus potentially enabling a larger effect of exogenously given GLP1-RA [34]. The results from the present study are consistent with the published results of the only randomized study of liraglutide (3.0 mg, subcutaneous daily injection) versus placebo in patients with weight regain following Roux-en-Y gastric bypass. After 12 months, 67.2% of the patients in the liraglutide arm versus 4.4% in the placebo arm had lost at least 5% of body weight [22]. Despite the difference in study duration, a similar proportion (69.0%) of the patients in our study achieved a weight loss of ≥5%.
As alluded to above, weight loss with GLP1-RA in clinical studies has been consistently shown to be smaller with liraglutide vs semaglutide [25, 26, 35]. In line with this, our sensitivity analysis showed a significantly greater magnitude of weight loss with semaglutide as compared to liraglutide (Fig. 4). Important to note though is that the greater effect on weight loss with semaglutide was observed despite liraglutide in 28 out of 29 patients being administered in the higher dose approved for weight reduction (3.0 mg, daily subcutaneous injection), whereas semaglutide was administered at the lower dose approved for T2D (1.0 mg, weekly subcutaneous injection or 14 mg, daily oral intake). Given the different indications, a randomized head-to-head comparison between liraglutide 3.0 mg, daily subcutaneous injection, and semaglutide 1.0 mg, weekly subcutaneous injection has not been done. In the STEP8 trial, comparing semaglutide (2.4 mg, weekly subcutaneous injection) with liraglutide (3.0 mg, daily subcutaneous injection) in patients with overweight or obesity and no previous bariatric surgery or T2D, a mean weight change from baseline of −15.8% with semaglutide vs −6.4% with liraglutide after 68 weeks was reported [27]. Taking in consideration the shorter duration and lower dose of semaglutide, the 34% greater weight loss with semaglutide as compared to liraglutide seen in the current study is in line with previous clinical studies. The higher proportion of self-payers among patients using semaglutide vs liraglutide (100 and 55.2%, respectively) could have positively influenced the weight outcome for semaglutide, since self-payer status tends to be associated with better adherence to treatment. However, a sensitivity analysis among patient treated with liraglutide showed no significant difference in weight loss between non-self-payers and self-payers, indicating that self-payer status likely did not significantly influence the outcome.
Furthermore, a tendency to a greater weight loss when GLP1-RA was initiated more than 72 months after bariatric surgery was observed (Supplement). The endocrine changes that take place after bariatric surgery are complex and not well understood. The rapid delivery of nutrients to the L-cells in the distal small intestine and colon after bariatric surgery leads to increased secretion of gut hormones, especially postprandial GLP-1 and peptide YY. Both hormones are known to reduce food intake by stimulating satiety and decreasing appetite, which most likely plays a central role for the weight loss effect of bariatric surgery [34]. Some studies indicate that the observed hormonal changes after bariatric surgery might wane with time [36], which may be associated with the development of weight regain. A study by Santo et al. demonstrated decreased postprandial levels of glucose-dependent insulinotropic polypeptide and GLP-1 in patients with weight regain after gastric bypass as compared to patients with favourable postoperative weight outcomes [37]. With the elevated endogenous GLP-1 secretion after bariatric surgery fading with time, exogenous stimulation of GLP-1 receptors with GLP1-RA could have a greater effect on weight.
Although previous studies have shown that GLP1-RAs tend to induce less weight loss in patients with than without T2D [29, 30, 32, 33], no statistically significant difference was observed in this study (Supplement). This is most likely due to the small number of patients with T2D (n=9) of which only two (22.2%) were treated with insulin. Insulin is an anabolic hormone and treatment is usually associated with weight gain, thus counteracting the weight loss effect of concomitant treatment with GLP1-RA. Based on the sensitivity analysis, the magnitude of weight regain did not seem to influence the effect of GLP1-RA on weight loss. The presence of a potential anatomical cause for weight regain did also not significantly change the efficacy on body weight loss of GLP1-RA, which supports the use of GLP1-RA in patients with such anatomical changes.
A clear consensus of what constitutes weight regain has not yet been defined [38, 39]. For the current study, we simply included patients at our bariatric centre for which GLP1-RA had been initiated due to weight regain after bariatric surgery, without further defining “weight regain.” We chose this approach as it realistically reflects the real-world clinical scenario where physicians take the overall weight status, e.g. BMI, cardiovascular risk factors, and patient preference into consideration.
Three patients did not lose weight after 6 months of GLP1-RA therapy, one female and two males, none of which had T2D. One patient had undergone sleeve gastrectomy, the other two proximal Roux-en-Y gastric bypass surgery. The total body weight increase after 6 months GLP1-RA therapy (liraglutide, 3.0 mg daily subcutaneous injection) was 1.3, 2.9, and 6.2%, respectively. One of these patients were subsequently diagnosed with a dilated pouch, which was surgically revised, and one patient received a surgical conversion from sleeve gastrectomy to proximal Roux-en-Y gastric bypass, although the efficacy on body weight of this approach is debatable. The third patient had no further medical or surgical intervention.
The reported adverse events when implementing GLP1-RAs in this patient population were of gastrointestinal nature, mild and transient. This is in line with the proven safety profile of most GLP1-RAs, including results from large cardiorenal outcome studies in patients without previous bariatric surgery [6,7,8]. A limitation of this approach is the lack of a standardized reporting procedure, which might have caused bias in the documented safety-related data, particularly with respect to the prevalence of transient mild events which might be underreported.
To the best of our knowledge, this is the first observational study of GLP1-RAs including both liraglutide and semaglutide in the treatment of weight regain after bariatric surgery. A strength of the study is the real-world setting, giving physicians and patients a realistic picture of what can be expected from GLP1-RA therapy in a similar setting. The retrospective observational study design comes with several limitations. The lack of randomization allows for selection bias and confounding. The heterogeneity of the patients concerning type and number of previous bariatric surgeries, the time span after bariatric surgery until GLP1-RA therapy initiation, the magnitude of weight regain, the GLP1-RA therapies given, and the possibility of an anatomical cause for weight gain following surgery among 20% of the patients certainly are a weakness of the study. On the other hand, demonstrating a significant weight loss with GLP1-RA therapy in line with previous clinical trial results, despite the heterogeneity of this real-world patient population, provides further support for the efficacy of implementing GLP1-RAs in this patient population. Since only approximately 60% of the patients at our centre that were prescribed or considered eligible for GLP1-RA therapy for weight regain after bariatric surgery were included in the present analysis, the weight lowering effect might be overestimated as patients who stopped treatment because of adverse side effects are not adequately represented. Nevertheless, the patients that completed 6 months of treatment with GLP1-RA demonstrated a considerable weight loss effect and highlight that GLP1-RA has the potential to offer substantial benefits to a considerable proportion of patients with weight regain after bariatric surgery, if available.
In conclusion, the results from the present retrospective observational study support the use of both liraglutide and semaglutide for the treatment of weight regain after bariatric surgery and emphasize the need for randomized clinical studies to confirm these results.
Data Availability
The dataset analysed in the current study is not publicly available as this is not approved by the participants within the framework of the general informed consent.
References
Syn NL, Cummings DE, Wang LZ, Lin DJ, Zhao JJ, Loh M, Koh ZJ, Chew CA, Loo YE, Tai BC, Kim G, So JBY, Kaplan LM, Dixon JB, Shabbir A. Association of metabolic–bariatric surgery with long-term survival in adults with and without diabetes: a one-stage meta-analysis of matched cohort and prospective controlled studies with 174 772 participants. The Lancet. 2021;397(10287):1830–41.
Voorwinde V, Steenhuis IHM, Janssen IMC, Monpellier VM, van Stralen MM. Definitions of long-term weight regain and their associations with clinical outcomes. Obes Surg. 2020;30(2):527–36.
Arterburn DE, Telem DA, Kushner RF, Courcoulas AP. Benefits and risks of bariatric surgery in adults: a review. JAMA. 2020;324(9):879.
Cambi MPC, Baretta GAP, Magro DDO, Boguszewski CL, Ribeiro IB, Jirapinyo P, de Moura DTH. Multidisciplinary approach for weight regain—how to manage this challenging condition: an expert review. Obes Surg. 2021;31(3):1290–303.
Shukla AP, He D, Saunders KH, Andrew C, Aronne LJ. Current concepts in management of weight regain following bariatric surgery. Expert Rev Endocrinol Metab. 2018;13(2):67–76.
Marso SP, Daniels GH, Brown-Frandsen K, Kristensen P, Mann JFE, Nauck MA, Nissen SE, Pocock S, Poulter NR, Ravn LS, Steinberg WM, Stockner M, Zinman B, Bergenstal RM, Buse JB. Liraglutide and cardiovascular outcomes in type 2 diabetes. N Engl J Med. 2016;375(4):311–22.
Marso SP, Bain SC, Consoli A, Eliaschewitz FG, Jódar E, Leiter LA, Lingvay I, Rosenstock J, Seufert J, Warren ML, Woo V, Hansen O, Holst AG, Pettersson J, Vilsbøll T. Semaglutide and cardiovascular outcomes in patients with type 2 diabetes. N Engl J Med. 2016;375(19):1834–44.
Kristensen SL, Rørth R, Jhund PS, Docherty KF, Sattar N, Preiss D, Køber L, Petrie MC, McMurray JJV. Cardiovascular, mortality, and kidney outcomes with GLP-1 receptor agonists in patients with type 2 diabetes: a systematic review and meta-analysis of cardiovascular outcome trials. Lancet Diabetes Endocrinol. 2019;7(10):776–85.
Rothkopf MM, Bilof ML, Haverstick LP, Nusbaum MJ. Synergistic weight loss and diabetes resolution with exenatide administration after laparoscopic gastric banding. Surg Obes Relat Dis. 2009;5(1):128–31.
Pajecki D, Halpern A, Cercato C, Mancini M, de Cleva R, Santo MA. Tratamento de curto prazo com liraglutide no reganho de peso após cirurgia bariátrica. Rev Colégio Bras Cir. 2013;40(3):191–5.
Gorgojo-Martínez JJ, Feo-Ortega G, Serrano-Moreno C. Effectiveness and tolerability of liraglutide in patients with type 2 diabetes mellitus and obesity after bariatric surgery. Surg Obes Relat Dis. 2016;12(10):1856–63.
Creange C, Lin E, Ren-Fielding C, Lofton H. Use of liraglutide for weight loss in patients with prior bariatric surgery. Surg Obes Relat Dis. 2016;12(7):S157.
Rye P, Modi R, Cawsey S, Sharma AM. Efficacy of high-dose liraglutide as an adjunct for weight loss in patients with prior bariatric surgery. Obes Surg. 2018;28(11):3553–8.
Wharton S, Kuk JL, Luszczynski M, et al. Liraglutide 3.0 mg for the management of insufficient weight loss or excessive weight regain post‐bariatric surgery. Clin Obes. 2019;9(4):e12323.
Suliman M, Buckley A, Al Tikriti A, Tan T, le Roux CW, Lessan N, Barakat M. Routine clinical use of liraglutide 3 mg for the treatment of obesity: outcomes in non-surgical and bariatric surgery patients. Diabetes Obes Metab. 2019;21(6):1498–501.
Bretault M, Carette C, Zaharia R, Vychnevskaia K, Bouillot JL, Czernichow S, Raffin-Sanson ML. Liraglutide 3 mg as a weight-loss strategy after failed bariatric surgery in a patient with hypothalamic obesity following craniopharyngioma. Diabetes Metab. 2020;46(6):514–5.
Rubio MA, Ramos-Leví AM. Initial experience with alternate-day liraglutide for weight regain following bariatric surgery. Obes Surg. 2021;31(9):4216–8.
Horber FF, Steffen R. Reversal of long-term weight regain after Roux-en-Y gastric bypass using liraglutide or surgical revision. A Prospective Study. Obes Surg. 2021;31(1):93–100.
Elhag W, El Ansari W. Effectiveness and safety of liraglutide in managing inadequate weight loss and weight regain after primary and revisional bariatric surgery: anthropometric and cardiometabolic outcomes. Obes Surg. 2022;32(4):1005–15.
Dharmaratnam VM, Lim E, Eng A, et al. Revisional surgery or pharmacotherapy for insufficient weight loss and weight regain after primary bariatric procedure: a descriptive study. Obes Surg. 2022;32(10):3298–3304.
Miras AD, Pérez-Pevida B, Aldhwayan M, Kamocka A, McGlone ER, Al-Najim W, Chahal H, Batterham RL, McGowan B, Khan O, Greener V, Ahmed AR, Petrie A, Scholtz S, Bloom SR, Tan TM. Adjunctive liraglutide treatment in patients with persistent or recurrent type 2 diabetes after metabolic surgery (GRAVITAS): a randomised, double-blind, placebo-controlled trial. Lancet Diabetes Endocrinol. 2019;7(7):549–59.
Clinical Efficacy and safety of using 3.0mg liraglutide to treat weight regain after Roux-en-Y gastric bypass surgery. [Internet]. [cited 2022 Oct 15]. Available from: https://www.clinicaltrials.gov/ct2/show/NCT03048578
Redmond IP, Shukla AP, Aronne LJ. Use of weight loss medications in patients after bariatric surgery. Curr Obes Rep. 2021;10(2):81–9.
Lautenbach A, Wernecke M, Huber TB, et al. The potential of semaglutide once-weekly in patients without type 2 diabetes with weight regain or insufficient weight loss after bariatric surgery—a retrospective analysis. Obes Surg. 2022;32(10):3280–3288.
O’Neil PM, Birkenfeld AL, McGowan B, Mosenzon O, Pedersen SD, Wharton S, Carson CG, Jepsen CH, Kabisch M, Wilding JPH. Efficacy and safety of semaglutide compared with liraglutide and placebo for weight loss in patients with obesity: a randomised, double-blind, placebo and active controlled, dose-ranging, phase 2 trial. The Lancet. 2018;392(10148):637–49.
Pratley R, Amod A, Hoff ST, Kadowaki T, Lingvay I, Nauck M, Pedersen KB, Saugstrup T, Meier JJ. Oral semaglutide versus subcutaneous liraglutide and placebo in type 2 diabetes (PIONEER 4): a randomised, double-blind, phase 3a trial. The Lancet. 2019;394(10192):39–50.
Rubino DM, Greenway FL, Khalid U, O’Neil PM, Rosenstock J, Sørrig R, Wadden TA, Wizert A, Garvey WT, STEP 8 Investigators, Arauz-Pacheco C, Cannon K, Downey HJ, Fitz-Patrick D, Geohas J, Gerety G, Gilbert J, Hollander P, Klein E, Laufer K, O’Donnell P, Rosenblit P, Toth P. Effect of weekly subcutaneous semaglutide vs daily liraglutide on body weight in adults with overweight or obesity without diabetes: the STEP 8 randomized clinical trial. JAMA. 2022;327(2):138.
Fujioka K, O’Neil PM, Davies M, Greenway F, C.W. Lau D, Claudius B, Skjøth TV, Bjørn Jensen C, P.H. Wilding J. Early weight loss with liraglutide 3.0 mg predicts 1-year weight loss and is associated with improvements in clinical markers. Obesity. 2016;4(11):2278–88.
Pi-Sunyer X, Astrup A, Fujioka K, Greenway F, Halpern A, Krempf M, Lau DCW, le Roux CW, Violante Ortiz R, Jensen CB, Wilding JPH. A randomized, controlled trial of 3.0 mg of liraglutide in weight management. N Engl J Med. 2015;373(1):11–22.
Davies MJ, Bergenstal R, Bode B, Kushner RF, Lewin A, Skjøth TV, Andreasen AH, Jensen CB, DeFronzo RA, for the NN8022-1922 Study Group. Efficacy of liraglutide for weight loss among patients with type 2 diabetes: the SCALE diabetes randomized clinical trial. JAMA. 2015;314(7):687.
Sorli C, Harashima S, ichi, Tsoukas GM, Unger J, Karsbøl JD, Hansen T, Bain SC. Efficacy and safety of once-weekly semaglutide monotherapy versus placebo in patients with type 2 diabetes (SUSTAIN 1): a double-blind, randomised, placebo-controlled, parallel-group, multinational, multicentre phase 3a trial. Lancet Diabetes Endocrinol. 2017;5(4):251–60.
Wilding JPH, Batterham RL, Calanna S, Davies M, Van Gaal LF, Lingvay I, McGowan BM, Rosenstock J, Tran MTD, Wadden TA, Wharton S, Yokote K, Zeuthen N, Kushner RF. Once-weekly semaglutide in adults with overweight or obesity. N Engl J Med. 2021;384(11):989–1002.
Davies M, Færch L, Jeppesen OK, Pakseresht A, Pedersen SD, Perreault L, Rosenstock J, Shimomura I, Viljoen A, Wadden TA, Lingvay I. Semaglutide 2·4 mg once a week in adults with overweight or obesity, and type 2 diabetes (STEP 2): a randomised, double-blind, double-dummy, placebo-controlled, phase 3 trial. The Lancet. 2021;397(10278):971–84.
Papamargaritis D, le Roux CW. Do gut hormones contribute to weight loss and glycaemic outcomes after bariatric surgery? Nutrients. 2021;13(3):762.
Capehorn MS, Catarig AM, Furberg JK, Janez A, Price HC, Tadayon S, Vergès B, Marre M. Efficacy and safety of once-weekly semaglutide 1.0 mg vs once-daily liraglutide 1.2 mg as add-on to 1–3 oral antidiabetic drugs in subjects with type 2 diabetes (SUSTAIN 10). Diabetes Metab. 2020;46(2):100–9.
Lampropoulos C, Alexandrides T, Tsochatzis S, Kehagias D, Kehagias I. Are the changes in gastrointestinal hormone secretion necessary for the success of bariatric surgery? A Critical Review of the Literature. Obes Surg. 2021;31(10):4575–84.
Santo MA, Riccioppo D, Pajecki D, Kawamoto F, de Cleva R, Antonangelo L, Marçal L, Cecconello I. Weight regain after gastric bypass: influence of gut hormones. Obes Surg. 2016;26(5):919–25.
Nedelcu M, Khwaja HA, Rogula TG. Weight regain after bariatric surgery—how should it be defined? Surg Obes Relat Dis. 2016;12(5):1129–30.
Lauti M, Kularatna M, Hill AG, MacCormick AD. Weight regain following sleeve gastrectomy—a systematic review. Obes Surg. 2016;26(6):1326–34.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethics Approval and Consent to Participate
Written informed consent was obtained from all individual participants included in the study. The study was conducted in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. The study protocol was approved by the regional ethics committee.
Conflict of Interest
Anders Boisen Jensen is a previous employee of Novo Nordisk (2008–2010) and has received speaker honoraria and financial support for attending congresses from Novo Nordisk.
Frida Renström has no conflict of interest to declare.
Stefan Aczél has received speaker honoraria and financial support for attending congresses from Novo Nordisk.
Patrick Folie and Magdalena Biraima-Steinemann have received speaker honoraria from Novo Nordisk.
Felix Beuschlein has received financial support for attending symposia and for educational programmes by Novo Nordisk.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Key Points
• Weight regain after bariatric surgery is a frequently occurring clinical challenge.
• The role of GLP1-RAs in weight regain after bariatric surgery is not yet defined.
• Two-thirds of weight regain after bariatric surgery can be safely lost with GLP1-RAs.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Jensen, A.B., Renström, F., Aczél, S. et al. Efficacy of the Glucagon-Like Peptide-1 Receptor Agonists Liraglutide and Semaglutide for the Treatment of Weight Regain After Bariatric surgery: a Retrospective Observational Study. OBES SURG 33, 1017–1025 (2023). https://doi.org/10.1007/s11695-023-06484-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11695-023-06484-8