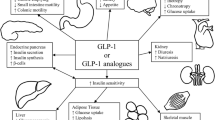
Abstract
Background
A significant number of patients face the issue of weight gain (WG) or inadequate weight loss (IWL) post-bariatric surgery for obesity. Several studies have been published evaluating the role of glucagon-like peptide-1 receptor agonists (GLP1RA) for weight loss post-bariatric surgery. However, no systematic review and meta-analysis (SRM) till date has evaluated the efficacy, safety and tolerability of GLP1RA in this clinical scenario. Hence, this SRM aimed to address this knowledge gap.
Methods
Databases were searched for randomized controlled trials (RCTs), case–control, cohort and observational studies involving use of GLP1RA in the intervention arm post-bariatric surgery. Primary outcome was weight loss post at least 3 months of therapy. Secondary outcomes were evaluation of body composition parameters, total adverse events (TAEs) and severe adverse events (SAEs).
Results
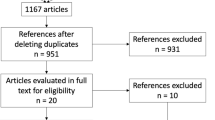
From initially screened 1759 articles, 8 studies (557 individuals) were analysed. Compared to placebo, patients receiving liraglutide had significantly greater weight loss after 6-month therapy [MD − 6.0 kg (95% CI, − 8.66 to − 3.33); P < 0.001; I2 = 79%]. Compared to liraglutide, semaglutide had significantly greater percent reduction in body weight after 6-month [MD − 2.57% (95% CI, − 3.91 to − 1.23); P < 0.001; I2 = 0%] and 12-month [MD − 4.15% (95% CI, − 6.96 to − 1.34); P = 0.004] therapy. In study by Murvelashvili et al. (2023), after 12-month therapy, semaglutide had significantly higher rates of achieving > 15% [OR 2.15 (95% CI, 1.07–4.33); P = 0.03; n = 207] and > 10% [OR 2.10 (95% CI, 1.19–3.71); P = 0.01; n = 207] weight loss. A significant decrease in fat mass [MD − 4.78 kg (95% CI, − 7.11 to − 2.45); P < 0.001], lean mass [MD − 3.01 kg (95% CI, − 4.80 to − 1.22); P = 0.001] and whole-body bone mineral density [MD − 0.02 kg/m2 (95% CI, − 0.04 to − 0.00); P = 0.03] was noted with liraglutide.
Conclusion
Current data is encouraging regarding use of GLP1RAs for managing WG or IWL post-bariatric surgery. Deterioration of bone health and muscle mass remains a concern needing further evaluation.
Trial Registration
The predefined protocol has been registered in PROSPERO having registration number of CRD42023473991.
Graphical Abstract




Similar content being viewed by others
References
Whitlock G, Lewington S, Sherliker P, et al. Body-mass index and cause-specific mortality in 900 000 adults: collaborative analyses of 57 prospective studies. Lancet. 2009;373:1083–96.
Lautenbach A, Kantowski T, Wagner J, et al. Sustained weight loss with semaglutide once weekly in patients without type 2 diabetes and post-bariatric treatment failure. Clin Obes. 2023;13(5): e12593. https://doi.org/10.1111/cob.12593.
Nedelcu M, Khwaja HA, Rogula TG. Weight regain after bariatric surgery-how should it be defined? Surg Obes Relat Dis. 2016;12(5):1129–30.
El Ansari W, Elhag W. Weight regain and insufficient weight loss after bariatric surgery: definitions, prevalence, mechanisms, predictors, prevention and management strategies, and knowledge gaps-a scoping review. Obes Surg. 2021;31(4):1755–66.
Colin IM, Gérard KM. Once-weekly 2.4 mg semaglutide for weight management in obesity: a game changer? touchREV Endocrinol. 2022;18(1):35–42.
Pi-Sunyer X, Astrup A, Fujioka K, et al. A randomized, controlled trial of 3.0 mg of liraglutide in weight management. N Engl J Med. 2015;373:11–22.
Rubino D, Abrahamsson N, Davies M, et al. Effect of continued weekly subcutaneous semaglutide vs placebo on weight loss maintenance in adults with overweight or obesity: the STEP 4 randomized clinical trial. JAMA. 2021;325:1414–25.
Garvey WT, Batterham RL, Bhatta M, et al. Two-year effect of semaglutide 2.4 mg vs placebo in adults with overweight or obesity: STEP 5. Obesity. 2021;29:43 (Oral abstract# 96).
Miras AD, Pérez-Pevida B, Aldhwayan M, et al. Adjunctive liraglutide treatment in patients with persistent or recurrent type 2 diabetes after metabolic surgery (GRAVITAS): a randomised, double-blind, placebo-controlled trial. Lancet Diabetes Endocrinol. 2019;7(7):549–59.
Jensen AB, Renström F, Aczél S, et al. Efficacy of the glucagon-like peptide-1 receptor agonists liraglutide and semaglutide for the treatment of weight regain after bariatric surgery: a retrospective observational study. Obes Surg. 2023;33(4):1017–25.
Murvelashvili N, Xie L, Schellinger JN, et al. Effectiveness of semaglutide versus liraglutide for treating post-metabolic and bariatric surgery weight recurrence. Obesity (Silver Spring). 2023;31(5):1280–9.
Mok J, Adeleke MO, Brown A, et al. Safety and efficacy of liraglutide, 3.0 mg, once daily vs placebo in patients with poor weight loss following metabolic surgery: the BARI-OPTIMISE randomized clinical trial. JAMA Surg. 2023;158(10):1003–11.
Coelho C, Dobbie LJ, Crane J, et al. Laparoscopic adjustable gastric banding with liraglutide in adults with obesity and type 2 diabetes (GLIDE): a pilot randomised placebo controlled trial. Int J Obes (Lond). 2023. https://doi.org/10.1038/s41366-023-01368-4.
Thakur U, Bhansali A, Gupta R, et al. Liraglutide augments weight loss after laparoscopic sleeve gastrectomy: a randomised, double-blind, placebo-control study. Obes Surg. 2021;31(1):84–92. https://doi.org/10.1007/s11695-020-04850-4.
Mosli MM, Elyas M. Does combining liraglutide with intragastric balloon insertion improve sustained weight reduction? Saudi J Gastroenterol. 2017;23(2):117–22. https://doi.org/10.4103/1319-3767.203362.
Hany M, Torensma B, Ibrahim M, et al Boosting weight loss after conversional RYGB with liraglutide and placebo use. A double-blind- randomized controlled trial. Int J Surg. 2023 Dec 14. https://doi.org/10.1097/JS9.0000000000000990.
Higgins JPT, Altman DG, Gøtzsche PC, et al. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ. 2011;18(343): d5928.
Dutta D, Bhattacharya S, Surana V, et al. Efficacy and safety of saroglitazar in managing hypertriglyceridemia in type-2 diabetes: a meta-analysis. Diabetes Metab Syndr. 2020;14(6):1759–68.
Dutta D, Agarwal A, Maisnam I, et al. Efficacy and safety of the novel dipeptidyl peptidase-4 inhibitor gemigliptin in the management of type 2 diabetes: a meta-analysis. Endocrinol Metab Seoul Korea. 2021;36(2):374–87.
Liberati A, Altman DG, Tetzlaff J, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate healthcare interventions: explanation and elaboration. BMJ. 2009;21(339): b2700.
Guyatt GH, Oxman AD, Vist GE, et al. GRADE: an emerging consensus on rating quality of evidence and strength of recommendations. BMJ. 2008;336(7650):924–6.
Song F, Eastwood AJ, Gilbody S, et al. Publication and related biases. Health Technol Assess Winch Engl. 2000;4(10):1–115.
Pajecki D, Halpern A, Cercato C, Mancini M, de Cleva R, Santo MA. Short-term use of liraglutide in the management of patients with weight regain after bariatric surgery. Rev Col Bras Cir. 2013;40(3):191–5.
Wharton S, Kuk JL, Luszczynski M, et al. Liraglutide 3.0 mg for the management of insufficient weight loss or excessive weight regain post-bariatric surgery. Clin Obes. 2019;9(4):e12323. https://doi.org/10.1111/cob.12323.
Muratori F, Vignati F, Di Sacco G, et al. Efficacy of liraglutide 3.0 mg treatment on weight loss in patients with weight regain after bariatric surgery. Eat Weight Disord. 2022;27(7):2775–81.
Colbourne JRM, Fisher OM, Mo S, et al. The role of adjuvant pharmacotherapy with liraglutide for patients with inadequate weight loss following bariatric surgery. Langenbecks Arch Surg. 2023;408(1):115. https://doi.org/10.1007/s00423-023-02805-8.
Elhag W, El Ansari W. Effectiveness and safety of liraglutide in managing inadequate weight loss and weight regain after primary and revisional bariatric surgery: anthropometric and cardiometabolic outcomes. Obes Surg. 2022;32(4):1005–15.
Gorgojo-Martínez JJ, Feo-Ortega G, Serrano-Moreno C. Effectiveness and tolerability of liraglutide in patients with type 2 diabetes mellitus and obesity after bariatric surgery. Surg Obes Relat Dis. 2016;12(10):1856–63.
Rye P, Modi R, Cawsey S, et al. Efficacy of high-dose liraglutide as an adjunct for weight loss in patients with prior bariatric surgery. Obes Surg. 2018;28(11):3553–8.
Rubio MA, Ramos-Leví AM. Initial experience with alternate-day liraglutide for weight regain following bariatric surgery. Obes Surg. 2021;31(9):4216–8.
Dharmaratnam VM, Lim E, Eng A, et al. Revisional surgery or pharmacotherapy for insufficient weight loss and weight regain after primary bariatric procedure: a descriptive study. Obes Surg. 2022;32(10):3298–304.
Suliman M, Buckley A, Al Tikriti A, et al. Routine clinical use of liraglutide 3 mg for the treatment of obesity: Outcomes in non-surgical and bariatric surgery patients. Diabetes Obes Metab. 2019;21(6):1498–501. https://doi.org/10.1111/dom.13672.
Kanai R, Kinoshita S, Kanbe I, et al. Once-weekly semaglutide administered after laparoscopic sleeve gastrectomy: effects on body weight, glycemic control, and measured nutritional metrics in Japanese patients having both obesity and type 2 diabetes. Obes Pillars. 2024;3(9): 100098. https://doi.org/10.1016/j.obpill.2023.100098.
Lautenbach A, Wernecke M, Huber TB, et al. The potential of semaglutide once-weekly in patients without type 2 diabetes with weight regain or insufficient weight loss after bariatric surgery-a retrospective analysis. Obes Surg. 2022;32(10):3280–8.
Maisnam I, Dutta D, Mukhopadhyay S, et al. Lean mass is the strongest predictor of bone mineral content in type-2 diabetes and normal individuals: an eastern India perspective. J Diabetes Metab Disord. 2014;13(1):90. https://doi.org/10.1186/s40200-014-0090-5.
Mozaffarian D. GLP-1 Agonists for obesity—a new recipe for success? JAMA. Published online February 29, 2024. https://doi.org/10.1001/jama.2024.2252.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Highlights
• Glucagon-like peptide-1 receptor agonists are effective for weight loss in post-bariatric surgery.
• Six-month liraglutide therapy caused greater weight loss compared to placebo [MD − 6.0kg].
• Six-month semaglutide caused significantly greater percent reduction weight compared to liraglutide [MD − 2.57%].
• Weight loss–related bone mineral and muscle loss remain a concern.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Dutta, D., Nagendra, L., Joshi, A. et al. Glucagon-Like Peptide-1 Receptor Agonists in Post-bariatric Surgery Patients: A Systematic Review and Meta-analysis. OBES SURG 34, 1653–1664 (2024). https://doi.org/10.1007/s11695-024-07175-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11695-024-07175-8