Abstract
Background
Short-chain fatty acids (SCFAs) and gut microbiota have health-related effects and are associated with a wide range of disorders. However, the changes of SCFAs and their receptors after sleeve gastrectomy (SG) remain unclear. This study aimed to examine changes of SCFAs and their receptors after SG in an obese rat model.
Methods
Thirty obese Sprague–Dawley rats eating a high-energy diet for 6 weeks were divided into three groups: sham-operated (SO) control, pair-fed (PF) control, and SG group. Six weeks after the surgery, metabolic parameters, SCFA levels in the blood and stool, mRNA and protein expression of SCFA receptors in the ileum and epididymal fat, and gut microbiota were examined.
Results
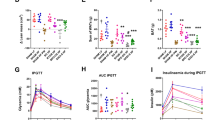
Metabolic parameters in the SG group were significantly improved compared with the SO group. Acetic acid levels in the blood and stool were significantly higher in the SG group than the PF group. The butyric acid level in the stool was also significantly higher in the SG group than in the PF group. In the ileum and epididymal fat, mRNA and protein expression of GPR41 was significantly higher in the SG group than in the other two groups, and mRNA and protein expression of GPR43 was significantly higher in the SG group than in the PF group. Increases in the genera Enterococcus, Lactobacillus, Lactococcus, and Clostridium were observed in the stool after SG.
Conclusions
SG may activate SCFA pathways through a change in gut microbiota.






Similar content being viewed by others
References
Samuel BS, Shaito A, Motoike T, et al. Effects of the gut microbiota on host adiposity are modulated by the short-chain fatty-acid binding G protein-coupled receptor, Gpr41. Proc Natl Acad Sci U S A. 2008;105:16767–72.
Vinolo MA, Rodrigues HG, Hatanaka E, Sato FT, Sampaio SC, Curi R. Suppressive effect of short-chain fatty acids on production of proinflammatory mediators by neutrophils. J Nutr Biochem. 2011;22:849–55.
Kasubuchi M, Hasegawa S, Hiramatsu T, Ichimura A, Kimura I. Dietary gut microbial metabolites, short-chain fatty acids, and host metabolic regulation. Nutrients. 2015;7:2839–49.
Tolhurst G, Heffron H, Lam YS, et al. Short-chain fatty acids stimulate glucagon-like peptide-1 secretion via the G-protein-coupled receptor FFAR2. Diabetes. 2012;61:364–71.
Brown AJ, Goldsworthy SM, Barnes AA, et al. The Orphan G protein-coupled receptors GPR41 and GPR43 are activated by propionate and other short chain carboxylic acids. J Biol Chem. 2003;278:11312–9.
Wu W, Sun M, Chen F, et al. Microbiota metabolite short-chain fatty acid acetate promotes intestinal IgA response to microbiota which is mediated by GPR43. Mucosal Immunol. 2017;10:946–56.
Macia L, Tan J, Vieira AT, et al. Metabolite-sensing receptors GPR43 and GPR109A facilitate dietary fibre-induced gut homeostasis through regulation of the inflammasome. Nat Commun. 2015;6:6734.
Tazoe H, Otomo Y, Karaki S, et al. Expression of short-chain fatty acid receptor GPR41 in the human colon. Biomed Res. 2009;30:149–56.
Nakajima A, Kaga N, Nakanishi Y, et al. Maternal high fiber diet during pregnancy and lactation influences regulatory T cell differentiation in offspring in mice. J Immunol. 2017;199:3516–24.
Furusawa Y, Obata Y, Fukuda S, et al. Commensal microbe-derived butyrate induces the differentiation of colonic regulatory T cells. Nature. 2013;504:446–50.
Sowah SA, Riedl L, Damms-Machado A, et al. Effects of weight-loss interventions on short-chain fatty acid concentrations in blood and feces of adults: a systematic review. Adv Nutr. 2019;10:673–84.
Angrisani L, Santonicola A, Iovino P, Ramos A, Shikora S, Kow L. Bariatric Surgery Survey 2018: similarities and disparities among the 5 IFSO chapters. Obes Surg. 2021;31:1937–48.
Ohta M, Kasama K, Sasaki A, et al. Current status of laparoscopic bariatric/metabolic surgery in Japan: the sixth nationwide survey by the Japan Consortium of Obesity and Metabolic Surgery. Asian J Endosc Surg. 2021;14:170–7.
Lutz TA, Bueter M. The use of rat and mouse models in bariatric surgery experiments. Front Nutr. 2016;3:25.
Takayama H, Ohta M, Tada K, et al. Additional effects of duodenojejunal bypass on glucose metabolism in a rat model of sleeve gastrectomy. Surg Today. 2019;49:637–44.
Lopez PP, Nicholson SE, Burkhardt GE, Johnson RA, Johnson FK. Development of a sleeve gastrectomy weight loss model in obese Zucker rats. J Surg Res. 2009;157:243–50.
Masuda T, Ohta M, Hirashita T, et al. A comparative study of gastric banding and sleeve gastrectomy in an obese diabetic rat model. Obes Surg. 2011;21:1774–80.
Jones DC, Kimeldorf DJ. Lifespan measurements in the male rat. J Gerontol. 1963;18:316–21.
Chambers AP, Jessen L, Ryan KK, et al. Weight-independent changes in blood glucose homeostasis after gastric bypass or vertical sleeve gastrectomy in rats. Gastroenterology. 2011;141:950–8.
Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC. Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia. 1985;28:412–9.
Tsukahara T, Matsukawa N, Tomonaga S, Inoue R, Ushida K, Ochiai K. High-sensitivity detection of short-chain fatty acids in porcine ileal, cecal, portal and abdominal blood by gas chromatography-mass spectrometry. Anim Sci J. 2014;85:494–8.
Tominaga M, Ohta M, Kai S, Iwaki K, Shibata K, Kitano S. Increased heat-shock protein 90 expression contributes to impaired adaptive cytoprotection in the gastric mucosa of portal hypertensive rats. J Gastroenterol Hepatol. 2009;24:1136–41.
Takahashi S, Tomita J, Nishioka K, Hisada T, Nishijima M. Development of a prokaryotic universal primer for simultaneous analysis of Bacteria and Archaea using next-generation sequencing. PLoS ONE. 2014;9: e105592.
Muyzer G, de Waal EC, Uitterlinden AG. Profiling of complex microbial populations by denaturing gradient gel electrophoresis analysis of polymerase chain reaction-amplified genes coding for 16S rRNA. Appl Environ Microbiol. 1993;59:695–700.
Hisada T, Endoh K, Kuriki K. Inter- and intra-individual variations in seasonal and daily stabilities of the human gut microbiota in Japanese. Arch Microbiol. 2015;197:919–34.
Wang Q, Garrity GM, Tiedje JM, Cole JR. Naive Bayesian classifier for rapid assignment of rRNA sequences into the new bacterial taxonomy. Appl Environ Microbiol. 2007;73:5261–7.
Kasai C, Sugimoto K, Moritani I, et al. Comparison of the gut microbiota composition between obese and non-obese individuals in a Japanese population, as analyzed by terminal restriction fragment length polymorphism and next-generation sequencing. BMC Gastroenterol. 2015;15:100.
Caporaso JG, Kuczynski J, Stombaugh J, et al. QIIME allows analysis of high-throughput community sequencing data. Nat Methods. 2010;7:335–6.
Russell WR, Gratz SW, Duncan SH, et al. High-protein, reduced-carbohydrate weight-loss diets promote metabolite profiles likely to be detrimental to colonic health. Am J Clin Nutr. 2011;93:1062–72.
Li Z, Yi CX, Katiraei S, et al. Butyrate reduces appetite and activates brown adipose tissue via the gut-brain neural circuit. Gut. 2018;67:1269–79.
Kimura I, Inoue D, Maeda T, et al. Short-chain fatty acids and ketones directly regulate sympathetic nervous system via G protein-coupled receptor 41 (GPR41). Proc Natl Acad Sci U S A. 2011;108:8030–5.
Hong YH, Nishimura Y, Hishikawa D, et al. Acetate and propionate short chain fatty acids stimulate adipogenesis via GPCR43. Endocrinology. 2005;146:5092–9.
Tang C, Ahmed K, Gille A, et al. Loss of FFA2 and FFA3 increases insulin secretion and improves glucose tolerance in type 2 diabetes. Nat Med. 2015;21:173–7.
Schwiertz A, Taras D, Schäfer K, et al. Microbiota and SCFA in lean and overweight healthy subjects. Obesity (Silver Spring). 2010;18:190–5.
Lu Y, Fan C, Li P, Lu Y, Chang X, Qi K. Short chain fatty acids prevent high-fat-diet-induced obesity in mice by regulating G protein-coupled receptors and gut microbiota. Sci Rep. 2016;6:37589.
Peiris M, Aktar R, Raynel S, et al. Effects of obesity and gastric bypass surgery on nutrient sensors, endocrine cells, and mucosal innervation of the mouse colon. Nutrients. 2018;10:1529.
Yu X, Wu Z, Song Z, et al. Single-anastomosis duodenal jejunal bypass improve glucose metabolism by regulating gut microbiota and short-chain fatty acids in Goto-Kakisaki rats. Front Microbiol. 2020;11:273.
Buchman AL, Scolapio J, Fryer J. AGA technical review on short bowel syndrome and intestinal transplantation. Gastroenterology. 2003;124:1111–34.
Smit G, Smit BA, Engels WJ. Flavour formation by lactic acid bacteria and biochemical flavour profiling of cheese products. FEMS Microbiol Rev. 2005;29:591–610.
Celiberto LS, Bedani R, Dejani NN, et al. Effect of a probiotic beverage consumption (Enterococcus faecium CRL 183 and Bifidobacterium longum ATCC 15707) in rats with chemically induced colitis. PLoS ONE. 2017;12: e0175935.
Cassir N, Benamar S, La Scola B. Clostridium butyricum: from beneficial to a new emerging pathogen. Clin Microbiol Infect. 2016;22:37–45.
Damms-Machado A, Mitra S, Schollenberger AE, et al. Effects of surgical and dietary weight loss therapy for obesity on gut microbiota composition and nutrient absorption. Biomed Res Int. 2015;2015: 806248.
Murphy R, Tsai P, Jüllig M, Liu A, Plank L, Booth M. Differential changes in gut microbiota after gastric bypass and sleeve gastrectomy bariatric surgery vary according to diabetes remission. Obes Surg. 2017;27:917–25.
Guo Y, Huang ZP, Liu CQ, Qi L, Sheng Y, Zou DJ. Modulation of the gut microbiome: a systematic review of the effect of bariatric surgery. Eur J Endocrinol. 2018;178:43–56.
Shah S, Shah P, Todkar J, Gagner M, Sonar S, Solav S. Prospective controlled study of effect of laparoscopic sleeve gastrectomy on small bowel transit time and gastric emptying half-time in morbidly obese patients with type 2 diabetes mellitus. Surg Obes Relat Dis. 2010;6:152–7.
Muredda L, Kępczyńska MA, Zaibi MS, Alomar SY, Trayhurn P. IL-1β and TNFα inhibit GPR120 (FFAR4) and stimulate GPR84 (EX33) and GPR41 (FFAR3) fatty acid receptor expression in human adipocytes: implications for the anti-inflammatory action of n-3 fatty acids. Arch Physiol Biochem. 2018;124:97–108.
Kimura I, Ichimura A, Ohue-Kitano R, Igarashi M. Free fatty acid receptors in health and disease. Physiol Rev. 2020;100:171–210.
Kimura I, Ozawa K, Inoue D, et al. The gut microbiota suppresses insulin-mediated fat accumulation via the short-chain fatty acid receptor GPR43. Nat Commun. 2013;4:1829.
Buchwald H. Metabolic surgery: a brief history and perspective. Surg Obes Relat Dis. 2010;6:221–2.
Sista F, Abruzzese V, Clementi M, Carandina S, Cecilia M, Amicucci G. The effect of sleeve gastrectomy on GLP-1 secretion and gastric emptying: a prospective study. Surg Obes Relat Dis. 2017;13:7–14.
Acknowledgements
We would like to thank Mr. Taisei Ryuo, Ms. Akari Himeda, and Ms. Nozomi Ito for their technical assistance with the experiments.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethical Approval
This study was approved by the Animal Committee of Oita University (#192002B).
Conflict of Interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Key Points
• Acetic acid levels in blood and stool were significantly higher in the SG vs. PF.
• Butyric acid level in the stool was significantly higher in the SG vs. PF.
• GPR41 and GPR43 in the ileum and epididymal fat were significantly higher in the SG vs. PF.
Rights and permissions
About this article
Cite this article
Fujinaga, A., Ohta, M., Endo, Y. et al. Changes of Short-Chain Fatty Acids and Their Receptors in an Obese Rat Model After Sleeve Gastrectomy. OBES SURG 32, 2649–2657 (2022). https://doi.org/10.1007/s11695-022-06130-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11695-022-06130-9