Abstract
Background
The protective effect of transit bipartition against esophagitis has not yet been proven. Thus, we investigate and compare the bariatric outcomes and esophagus’ histological changes of sleeve gastrectomy (SG), SG with transit bipartition (SG-TB), and the proximal SG-TB (SG-PTB) in a rodent model.
Methods
This study included 45 diabetic Sprague–Dawley rats assigned to one of the four groups, SG-PTB (n = 15), SG-TB (n = 12), SG (n = 10), and SHAM (n = 8). Eight surviving rats from each group were included for further investigation. Histological analysis of the gastroesophageal junction was performed. Body weight, food intake, glucose control, and hormonal changes (glucagon-like peptide-1 and insulin) were assessed before and after surgery in all groups.
Results
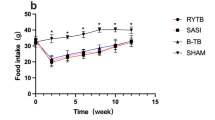
Preoperatively, no significant differences were observed in food intake, body weight, and fasting blood glucose levels among the groups. Postoperatively, the SG-PTB and SG-TB groups showed significantly superior glucose control compared to the SG group following the gavage of glucose (p < 0.05). Postoperatively, the SG-PTB and SG-TB groups had higher postoperative GLP-1 levels than postoperative SG and SHAM groups. More severe esophageal hyperpapillomatosis (EHP) of the esophageal section was observed in the SG group. The mucosal height of the SG group was significantly higher than that of the SG-PTB, SG-TB, and SHAM groups (p < 0.05).
Conclusion
The transit bipartition procedure may protect the distal esophagus from histological changes associated with esophagitis. Clinical studies are needed to confirm the anti-reflux effects of transit bipartition.
Graphical abstract






Similar content being viewed by others
References
Santoro S, Malzoni CE, Velhote MC, Milleo FQ, Santo MA, Klajner S, Damiani D, Maksoud JG. Digestive Adaptation with Intestinal Reserve: a neuroendocrine-based operation for morbid obesity. Obes Surg. 2006 Oct;16(10):1371–9.
Yormaz S, Yılmaz H, Ece I, Sahin M. Laparoscopic ileal interposition with diverted sleeve gastrectomy versus laparoscopic transit bipartition with sleeve gastrectomy for better glycemic outcomes in T2DM patients. Obes Surg. 2018 Jan;28(1):77–86.
Cagiltay E, Celik A, Dixon JB, Pouwels S, Santoro S, Gupta A, Ugale S, Abdul-Ghani M. Effects of different metabolic states and surgical models on glucose metabolism and secretion of ileal L-cell peptides: results from the HIPER-1 study. Diabet Med. 2020 Apr;37(4):697–704.
Topart P, Becouarn G, Finel JB. Is transit bipartition a better alternative to biliopancreatic diversion with duodenal switch for superobesity? Comparison of the early results of both procedures. Surg Obes Relat Dis. 2020;16(4):497–502.
Topart P, Becouarn G, Finel JB. Comparison of 2-year results of Roux-en-Y gastric bypass and transit bipartition with sleeve gastrectomy for superobesity. Obes Surg. 2020;30(9):3402–7.
Ece I, Yilmaz H, Yormaz S, Çolak B, Calisir A, Sahin M. The short-term effects of transit bipartition with sleeve gastrectomy and distal-Roux-en-Y gastric bypass on glycemic control, weight loss, and nutritional status in morbidly obese and type 2 diabetes mellitus patients. Obes Surg. 2021 May;31(5):2062–71.
Welbourn R, Hollyman M, Kinsman R, et al. Bariatric surgery worldwide: baseline demographic description and one-year outcomes from the Fourth IFSO Global Registry Report 2018. Obes Surg. 2019;29(3):782–95.
Peterli R, Wölnerhanssen BK, Peters T, et al. Effect of laparoscopic sleeve gastrectomy vs laparoscopic Roux-en-Y gastric bypass on weight loss in patients with morbid obesity: the SM-BOSS Randomized Clinical Trial. JAMA. 2018 Jan 16;319(3):255–65.
Yeung KTD, Penney N, Ashrafian L, Darzi A, Ashrafian H. Does sleeve gastrectomy expose the distal esophagus to severe reflux?: A systematic review and meta-analysis. Ann Surg. 2020 Feb;271(2):257–65.
Genco A, Castagneto-Gissey L, Lorenzo M, Ernesti I, Soricelli E, Casella G. Esophageal adenocarcinoma after sleeve gastrectomy: actual or potential threat? Italian series and literature review. Surg Obes Relat Dis. 2021 May;17(5):848–54.
Felinska E, Billeter A, Nickel F, Contin P, Berlth F, Chand B, Grimminger P, Mikami D, Schoppmann SF, Müller-Stich B. Do we understand the pathophysiology of GERD after sleeve gastrectomy? Ann N Y Acad Sci. 2020 Dec;1482(1):26–35.
Santoro S, Castro LC, Velhote MC, et al. Sleeve gastrectomy with transit bipartition: a potent intervention for metabolic syndrome and obesity. Ann Surg. 2012;256(1):104–10.
Del Genio G, Tolone S, Limongelli P, et al. Sleeve gastrectomy and development of “de novo” gastroesophageal reflux. Obes Surg. 2014 Jan;24(1):71–7.
Altieri MS, Shroyer KR, Pryor A, Pagnotti GM, Ete Chan M, Talamini M, Telem DA. The association between sleeve gastrectomy and histopathologic changes consistent with esophagitis in a rodent model. Surg Obes Relat Dis. 2015;11(6):1289–94.
Widjaja J, Dolo PR, Zhang Q, et al. Bypassed and preserved stomach resulted in superior glucose control in Sprague-Dawley rats with streptozotocin-induced diabetes. Sci Rep. 2019;9(1):9981.
Liu P, Widjaja J, Dolo PR, Yao L, Hong J, Shao Y, Zhu X. Comparing the anti-diabetic effect of sleeve gastrectomy with transit bipartition against sleeve gastrectomy and Roux-en-Y gastric bypass using a diabetic rodent model. Obes Surg. 2021 May;31(5):2203–10.
Fisher OM, Chan DL, Talbot ML, et al. Barrett’s oesophagus and bariatric/metabolic surgery-IFSO 2020 position statement. Obes Surg. 2021 Mar;31(3):915–34.
Felsenreich DM, Kefurt R, Schermann M, Beckerhinn P, Kristo I, Krebs M, Prager G, Langer FB. Reflux, sleeve dilation, and Barrett’s esophagus after laparoscopic sleeve gastrectomy: long-term follow-up. Obes Surg. 2017 Dec;27(12):3092–101.
Qumseya BJ, Qumsiyeh Y, Ponniah SA, Estores D, Yang D, Johnson-Mann CN, Friedman J, Ayzengart A, Draganov PV. Barrett’s esophagus after sleeve gastrectomy: a systematic review and meta-analysis. Gastrointest Endosc. 2021 Feb;93(2):343-352.e2.
Mala T. The gastric remnant in Roux-en-Y gastric bypass: challenges and possibilities. J Clin Gastroenterol. 2016 Aug;50(7):527–31.
Tornese S, Aiolfi A, Bonitta G, Rausa E, Guerrazzi G, Bruni PG, Micheletto G, Bona D. Remnant gastric cancer after Roux-en-Y gastric bypass: narrative review of the literature. Obes Surg. 2019 Aug;29(8):2609–13.
Andreollo NA, Santos EF, Araújo MR, Lopes LR. Rat’s age versus human’s age: what is the relationship? Arq Bras Cir Dig. 2012;25(1):49–51.
Sengupta P. The laboratory rat: relating its age with human’s. Int J Prev Med. 2013 Jun;4(6):624–30.
Grice HC. Safety evaluation of butylated hydroxyanisole from the perspective of effects on forestomach and oesophageal squamous epithelium. Food Chem Toxicol. 1988 Aug;26(8):717–23.
Funding
This study was supported by the Science and Technology Program Project of Xuzhou (KC19157).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Statement of Informed Consent
Not applicable.
Statement of Animal Right
All applicable institutional and national guidelines of the People’s Republic of China for the care and use of animals were followed.
Conflict of Interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Key Points
• A more severe esophageal hyperpapillomatosis (EHP) of the esophageal section was observed in the sleeve gastrectomy (SG) group, but not in the sleeve gastrectomy with transit bipartition (SG-TB) and sleeve gastrectomy with proximal transit bipartition (SG-PTB).
• Compared to SG, SG-TB may protect the distal esophagus from the histological changes associated with esophagitis.
• There is no significant difference esophageal hyperpapillomatosis (EHP) of the esophageal section between the SG-TB and SG-PTB models.
Rights and permissions
About this article
Cite this article
Wang, M., Widjaja, J., Dolo, P.R. et al. The Protective Effect of Transit Bipartition and Its Modification Against Sleeve Gastrectomy-Related Esophagitis in a Rodent Model. OBES SURG 32, 1149–1156 (2022). https://doi.org/10.1007/s11695-022-05907-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11695-022-05907-2