Abstract
Background
Despite limited evidence about the efficacy and safety of anticoagulation in patients post bariatric surgery, both vitamin K antagonists (VKA) and direct-acting oral anticoagulants (DOACs) are commonly prescribed.
Aim
To evaluate plasma anti-Xa levels of DOACs in morbidly obese (MO) patients before and after a Roux-en-Y gastric bypass (RYGB) procedure.
Patients and Methods
Retrospective, cross-sectional, and longitudinal study of anti-Xa activity of apixaban or rivaroxaban in MO patients (N = 41).
Results
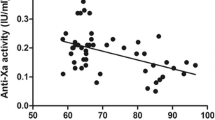
Preoperative analysis of plasma anti-Xa levels were within the normal range in patients using apixaban (n = 29; body mass index [BMI] 44.5 ± 5.1 kg/m2) as well as those using rivaroxaban (n = 12; BMI 42.6 ± 5.9 kg/m2). Postoperative anti-Xa levels of apixaban were all within the therapeutic range, whereas anti-Xa levels of rivaroxaban were subtherapeutic in nine out of 14 (64%) patients. Perioperative longitudinal follow-up in patients using apixaban (n = 18) showed no significant change in anti-Xa levels after RYGB.
Conclusion
Plasma anti-Xa levels of apixaban in MO patients remained within the therapeutic range up to a body weight of 144 kg. In patients using rivaroxaban, no statistically significant relation between anti-Xa levels and bodyweight was found. After RYGB, plasma anti-Xa levels of apixaban were unaffected, whereas plasma anti-Xa levels of rivaroxaban tended to become subtherapeutic.




Similar content being viewed by others
References
Vyas V, Lambiase P. Obesity and atrial fibrillation: epidemiology, pathophysiology and novel therapeutic opportunities. Arrhythm Electrophysiol Rev. 2019;8(1):28–36.
Ibáñez L, Sabaté M, Vidal X, et al. Incidence of direct oral anticoagulant use in patients with nonvalvular atrial fibrillation and characteristics of users in 6 European countries (2008–2015): a cross-national drug utilization study. Br J Clin Pharmacol. 2019;85(11):2524–39.
Yang G, De Staercke C, Hooper WC. The effects of obesity on venous thromboembolism: a review. Open J Prev Med. 2012;2(4):499–509.
Park J, Lee SR, Choi EK, et al. Effectiveness and safety of direct oral anticoagulant for secondary prevention in Asians with atrial fibrillation. J Clin Med. 2019;8(12).
Jong L, Koops M, Gout-Zwart JJ, et al. Trends in direct oral anticoagulant (DOAC) use: health benefits and patient preference. Neth J Med. 2018;76:426–30.
Chan N, Sobieraj-Teague M, Eikelboom JW. Direct oral anticoagulants: evidence and unresolved issues. Lancet. 2020;396(10264):1767–76.
Ruff CT, Giugliano RP, Braunwald E, et al. Comparison of the efficacy and safety of new oral anticoagulants with warfarin in patients with atrial fibrillation: a meta-analysis of randomised trials. Lancet. 2014;383(9921):955–62.
Ng DL, Gan GG, Chai CS, et al. Comparing quality of life and treatment satisfaction between patients on warfarin and direct oral anticoagulants: a cross-sectional study. Patient Prefer Adherence. 2019;13:1363–73.
Heidbuchel H, Verhamme P, Alings M, et al. Updated European Heart Rhythm Association Practical Guide on the use of non-vitamin K antagonist anticoagulants in patients with non-valvular atrial fibrillation. Europace. 2015;17(10):1467–507.
Kubitza D, Becka M, Zuehlsdorf M, et al. Effect of food, an antacid, and the H2 antagonist ranitidine on the absorption of BAY 59–7939 (rivaroxaban), an oral, direct factor Xa inhibitor, in healthy subjects. J Clin Pharmacol. 2006;46(5):549–58.
Song Y, Wang X, Perlstein I, et al. Relative bioavailability of apixaban solution or crushed tablet formulations administered by mouth or nasogastric tube in healthy subjects. Clin Ther. 2015;37(8):1703–12.
Frost C, Wang J, Nepal S, et al. Apixaban, an oral, direct factor Xa inhibitor: single dose safety, pharmacokinetics, pharmacodynamics and food effect in healthy subjects. Br J Clin Pharmacol. 2013;75(2):476–87.
Cada DJ, Levien TL, Baker DE. Apixaban. Hosp Pharm. 2013;48(6):494–509.
Sharma G, Hanipah ZN, Aminian A, et al. Bariatric surgery in patients on chronic anticoagulation therapy. Obes Surg. 2018;28(8):2225–32.
Sherf-Dagan S, Goldenshluger A, Azran C, et al. Vitamin K–what is known regarding bariatric surgery patients: a systematic review. Surg Obes Relat Dis. 2019;15(8):1402–13.
Homan J, Ruinemans-Koerts J, Aarts EO. Management of vitamin K deficiency after biliopancreatic diversion with or without duodenal switch. Surg Obes Relat Dis. 2016;12(2):338–44.
Kido K, Lee JC, Hellwig T, Gulseth MP. Use of direct oral anticoagulants in morbidly obese patients. Pharmacotherapy. 2020;40(1):72–83.
Upreti VV, Wang J, Barrett YC, et al. Effect of extremes of body weight on the pharmacokinetics, pharmacodynamics, safety and tolerability of apixaban in healthy subjects. Br J Clin Pharmacol. 2013;76(6):908–16.
Choi Y, Kushnir M, Billett HH. Apixaban is safe and effective in morbidly obese patients: a retrospective analysis of 390 patients with BMI ≥40. Blood. 2017;130(Supplement 1):1105.
Safouris A, Demulder A, Triantafyllou N, et al. Rivaroxaban presents a better pharmacokinetic profile than dabigatran in an obese non-diabetic stroke patient. J Neurol Sci. 2014;346(1–2):366–7.
Kubitza D, Becka M, Zuehlsdorf M, et al. Body weight has limited influence on the safety, tolerability, pharmacokinetics, or pharmacodynamics of rivaroxaban (BAY 59–7939) in healthy subjects. J Clin Pharmacol. 2007;47(2):218–26.
Patil T, Lebrecht M. A single center retrospective cohort study evaluating use of direct oral anticoagulants (DOACs) in morbidly obese veteran population. Thromb Res. 2020;192:124–30.
Rottenstreich A, Barkai A, Arad A, et al. The effect of bariatric surgery on direct-acting oral anticoagulant drug levels. Thromb Res. 2018;163:190–5.
Mahlmann A, Gehrisch S, Beyer-Westendorf J. Pharmacokinetics of rivaroxaban after bariatric surgery: a case report. J Thromb Thrombolysis. 2013;36(4):533–5.
Leven C, Hoffmann C, Roche C, et al. Impact of bariatric surgery on oral anticoagulants pharmacology, and consequences for clinical practice: a narrative review. Fundam Clin Pharmacol. 2021;35(1):53–61.
Byon W, Garonzik S, Boyd RA, et al. Apixaban: a clinical pharmacokinetic and pharmacodynamic review. Clin Pharmacokinet. 2019;58(10):1265–79.
Harder S. Pharmacokinetic and pharmacodynamic evaluation of rivaroxaban: considerations for the treatment of venous thromboembolism. Thromb J. 2014;12(1):22.
Testa S, Paoletti O, Legnani C, et al. Low drug levels and thrombotic complications in high-risk atrial fibrillation patients treated with direct oral anticoagulants. J Thromb Haemost. 2018;16(5):842–8.
Kröll D, Nett PC, Borbély YM, et al. The effect of bariatric surgery on the direct oral anticoagulant rivaroxaban: the extension study. Surg Obes Relat Dis. 2018;14(12):1890–6.
Author information
Authors and Affiliations
Contributions
Thom Kok conceived and designed the analysis; collected the data; contributed data or analysis tools; performed the analysis; and wrote the paper. Hans de Boer conceived and designed the analysis; contributed data or analysis tools; performed the analysis; and wrote the paper. Houshang Monajemi conceived and designed the analysis; contributed data or analysis tools; performed the analysis; and wrote the paper. Marcel Hovens wrote the paper. Bart Witteman wrote the paper. Matthijs van Luin wrote the paper.
Corresponding author
Ethics declarations
Ethics Approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed Consent
Informed consent was obtained from all individual participants included in the study.
Conflict of Interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Key Points
- There is no evidence of safe use for DOACs in morbid obesity (MO) and after bariatric surgery (BS).
- We performed a retrospective, cross-sectional, and longitudinal study of anti-Xa levels for apixaban and rivaroxaban for patients with MO and after BS.
- Despite small numbers, apixaban and rivaroxaban appear to be safe in MO, while apixaban shows in range anti-Xa levels after BS.
- Further investigation is needed, but apixaban could be a possible safe medication to use in this category.
Rights and permissions
About this article
Cite this article
Kok, T., de Boer, H., Witteman, B. et al. Anti-Xa Levels in Morbidly Obese Patients Using Apixaban or Rivaroxaban, Before and After Bariatric Surgery. OBES SURG 32, 607–614 (2022). https://doi.org/10.1007/s11695-021-05814-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11695-021-05814-y