Abstract
Introduction
The Orbera365 is a new balloon that can stay in the stomach for up to 12 months. The aim of this study is to investigate the safety and effect of Orbera365.
Method
Prospective study on our initial experience with a consecutive group of patients who underwent the insertion of Orbera365 in the period between September 2019 and August 2020. The patients were followed up to assess, pain, nausea, and vomiting after procedure, weight loss, and the complication rate.
Results
A total of 97 patients underwent Orbera365 placement. Mean weight and BMI before the procedure were 93.8 ± 15.2 kg and 35.2 ± 4.4 kg/m2, respectively, which dropped to 80.6 ± 13.1 kg and 29.8 ± 4.0 kg/m2 by 8.2 months and were 82.4 ± 16.1 and 30.4 ± 4.6 at the last day of follow-up of 12.9 months. Fourteen patients did not tolerate the balloon, and had to have it removed, six of them in the first week, and eight within the first 8 months of insertion. Other than intolerance, two patients had balloon rupture, three patients had leakage at time of insertion requiring balloon replacement, two patient had pancreatitis, one patient had spontaneous balloon hyperinflation, and one patient had balloon deflation and vomited the balloon. At day of last follow-up, total body weight loss % (TBWL%) was 16.2 ± 10.1 and %EWL was 54.6 ± 38.3.
Conclusion
Orbera365 is safe and effective for weight loss.
Graphical abstract

Similar content being viewed by others
Avoid common mistakes on your manuscript.
Background and Introduction
Obesity and its complications have been rising worldwide [1]. The prevalence of obesity (body mass index (BMI) ≥ 30 kg/m2) in Kuwait is 40.3%, one of the highest in the world [2]. Several interventions are available including lifestyle intervention, drug therapy, devices such as intragastric balloon (IGB) devices, and bariatric surgery [3]. Lifestyle modification is indicated for all patients with obesity but is often inadequate at achieving weight goals [4]. Drug therapies are increasingly available, however are not always well tolerated [5]. While bariatric surgery is the most effective method of weight reduction; it is invasive and is not indicated in some patients with obesity such as those with BMI < 35 kg/m2 without type II diabetes mellitus (T2DM) and those with a BMI of 35 to 40 kg/m2 without co-existing medical conditions [6]. IGB devices offer an additional option for patients who do not meet criteria for bariatric surgery but are unable to achieve adequate weight loss with lifestyle interventions and medical therapy alone.
IGB devices achieve weight reduction through early satiety and delaying gastric emptying [7]. They have been demonstrated to be effective and generally safe on the short term [8, 9]. Potential safety issues include intolerance due to gastrointestinal (GI) symptoms as well as gastric ulcers [8].
The methods of insertion and removal of balloons and their duration vary according to balloon type. The Orbera365 is a new balloon that is inserted by endoscopy and can remain in the stomach for 365 days and then is removed by endoscopy. In this retrospective, single-arm study, we reviewed the rate of complications in the initial series of patients who received the Orbera365 balloon for weight management. This is one of the earliest reports in the literature on this relatively new device and more studies are required to determine the safety, effectiveness, early deflation, device malfunction, tolerance, and pancreatitis.
Methods
The ORBERA365™ System
The Orbera365 intragastric balloon system (Apollo endosurgery, Austin, TX, USA) is a gastric balloon that is inserted under endoscopy and sedation or general anesthesia and filled with saline to obtain a spherical shape. It can be filled from 400 to 700 ml, and it contains a self-sealing valve that allows detachment from the external delivery device. That device basically consists of a 6.5 mm external diameter polyurethane catheter, with the end connected to a sheath where the collapsed balloon resides. In order to ensure the integrity of the balloon and reduce risks of ulcers, proton pump inhibitors are initiated as acid degrades the silicone elastomer. We initially perform a gastroscopy under general anesthesia prior to the balloon placement to ensure that the stomach and duodenum are free of pathology. We then insert the balloon and repeat the gastroscopy prior to filling the balloon. The volume we fill depends on the fundus size upon retroflexion. We fill the balloon until the wall of the balloon touches the wall of the stomach and presses on it. After filling the balloon and detaching the external delivery device, endoscopy is repeated.
We perform the removal of the balloon device under general anesthesia. We start with a gastroscopy and then use a needle to puncture the balloon to allow the suction tube to enter the balloon shell and suction the fluid out. The tube is then removed, and a grasper inserted through the gastroscope to pull the deflated balloon out. A gastroscopy is then repeated. We use the removal kit from Prince Medical consisting of the deflation needle of the balloon and the extraction forceps.
Study Design
This is the first-year consecutive experience of two obesity and metabolic surgeons MJ and SA with the Orbera365 in the period between September 2019 and August 2020. The patients were followed up with their surgeon who inserted the Orbera365 in the out-patient clinics and through phone interviews at time intervals of 1 month, 6 months, 8 months, and last day of follow-up. At the 1 month visit, all patients answered a questionnaire detailing the symptoms that occurred in the immediate period post insertion. At 6 months and 8 months visit, the weight was assessed. At the date of last follow-up, all patients were contacted through phone interviews with a short questionnaire assessing the current weight and post-procedural symptoms such as pain, nausea, and vomiting. The weight loss was calculated by applying percent total weight loss (%TWL) and percent excess weight loss (%EWL) equation. All patients were followed up by the dietician to administer a high protein low calorie diet.
Anthropometric Measurements
Anthropometric measurements included weight and height of all the subjects. The measurements were obtained on the first out-patient clinic visit and at 6 and 8 months post-insertion in the clinic. At the date of last follow-up, patients were contacted by phone interviews and reported their current weight.
Subjects and Inclusion Criteria
Males and females aged 18 years and above, with a minimum BMI of 27.5 kg/m2, were included. Each patient then was evaluated for eligibility for Orbera365 implantation. Those with any contraindications for Orbera365 placement, including eating disorders (bulimia nervosa and anorexia nervosa), history of Crohn’s disease, severe gastroesophageal reflux disease (GERD) with hiatal, bleeding disorders or patients on anticoagulation, history of varices, history of acute pancreatitis, pregnancy, and previous gastric surgery, were excluded. All patients were seen by the physician and the nutritionist prior to insertion and have undergone blood tests including complete blood count, renal profile, and thyroid function test.
Orbera365 Balloon Insertion
Patients fasted for at least 12 h prior to the procedure and received a single 125 mg per os (PO) dose of the anti-emetic Aprepitant (Emend®) 4 h before the deployment of the balloon. They also received intravenous proton pump inhibitor, dexamethasone 8 mg, ondansetron 8 mg, and Buscopan 20 mg. All balloons were inserted under general anesthesia and endotracheal intubation with the patient in a supine position. A gastroscopy is performed initially and then is removed to introduce the balloon. Gastroscopy is then repeated, and the balloon is filled under direct vision and after detachment of the external delivery tube to ensure no leakage of the balloon.
Post-insertion, patients were admitted under observation for 6 h to receive intravenous hydration. On discharge, they are prescribed Ondansetron 8 mg every 8 h, Metochlopramide 10 mg every 6 h, and paracetamol 1gm every 6 h for the first 48 h. A phone follow-up was instituted for all patients in the first 3 days post-insertion and if the patient were thought to be dehydrated with repeated nausea and vomiting, then IV hydration and IV antiemetics would be given. Omeprazole 40 mg daily was started 1 week prior to placement and was continued until the end of treatment. Fluid hydration was permitted for the first 24 h. During the first week, a gradual progression to a semi-liquid diet (yogurt, mashed potatoes, clear soup, puréed vegetables, and eggs) was recommended. At the beginning of the second week, the patient proceeded with caution to a hypocaloric, textured diet plan. Patients were encouraged to regularly exercise.
Statistical Analysis
Statistical analysis was performed using IBM SPSS Statistics v.25. Descriptive analysis was conducted, and frequencies are reported in Supplementary table 1–5.
Results
A total of 97 patients had the Orbera365 inserted. A total of 83 patients tolerated the Orbera365. Intolerance of the Orbera365 occurred in 6 patients within the first week and in 8 patients within the 1-year course of the balloon due to neo-onset of repeated nausea, vomiting with abdominal pain. Females represented 76 (78.4%) of our sample, while male represented 21 (21.6%). The mean age of patients was 31 years (Supplementary table 1). The mean weight and BMI of the patients before the Orbera365 insertion were 93.8 ± 15.2 kg and 35.2 ± 4.4 kg/m2, respectively.
Three patients (3%) had severe gastritis with negative Helicobacter pylori at time of endoscopy and therefore did not undergo placement and underwent a 6-week course of proton pump inhibitors (PPI) treatment prior to insertion at a second endoscopy. All these three patients tolerated the balloon without complications.
Post-procedural symptoms noticed by the patients were as follows: abdominal pain 51 (53%) and nausea and vomiting 62 (64%), in which 69 patients (75%) required intravenous hydration in the first 3 days post-insertion (Supplementary table 2). A total of 14 patients did not tolerate the balloon causing repeated nausea, vomiting, and pain and had to have it removed: six of them in the first week and eight in the coming 8 months post-insertion. None of the patients who had balloon intolerance had abnormal finding at endoscopy neither at time of insertion nor at time of removal except two patients who had it removed more than 1 week post-insertion, where ulceration was observed. Other than balloon intolerance, three patients had spontaneous balloon deflation, one patient noticed blue urine and stool 10 months after insertion but could not seek immediate medical help due to lockdown due to COVID-19, and a CT scan done a week after noticing the blue urine and stool showed the deflated balloon in the hepatic flexure (Fig. 1). She passed the balloon spontaneously without the need for intervention. The second patient with the balloon rupture also noticed blue urine and stool 6 months after insertion but did seek help and the balloon was retrieved from the stomach (Fig. 2). Another patient had balloon rupture and vomited the ruptured balloon. All three patients were not adherent to their PPI daily regimen.
Three patients had leakage at time of insertion requiring balloon replacement. In those patients, the external delivery device detached suddenly while filling the balloon without excessive movement explaining the detachment (Fig. 3). All these three cases occurred in July–August 2020 and required removal immediately and placement of new balloons.
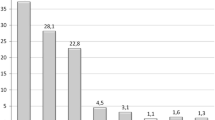
Two patient had pancreatitis, one 3 weeks post-insertion with an amylase of 1850 (Fig. 4), and the other 3 months post-insertion with an amylase of 973 with negative gallbladder ultrasound for stones, and both patients are abstinent from alcohol. We removed the balloon in both patients without further complications. One patient had spontaneous balloon hyperinflammation causing repeated nausea and vomiting, requiring removal of the balloon. All complications are summarized in Supplementary table 3. Mean weight and BMI before the procedure were 93.8 ± 15.2 kg and 35.2 ± 4.4 kg/m2, respectively, which dropped to 80.6 ± 13.1 kg and 29.8 ± 4.0 kg/m2 by 8.2 months and were 82.4 ± 16.1 and 30.4 ± 4.6 at the last day of follow-up of 12.9 months. At day of last follow-up, BMI change was − 5.9 ± 4.1 kg/m2, total body weight loss % (TBWL%) was 16.2 ± 10.1, and %EWL was 54.6 ± 38.3 (Fig. 5 and Supplementary table 4).
Discussion
In this study, evaluating the initial experience with the relatively new Orbera365, we found an overall higher than expected complication rate (23.7%). The most worrisome complication was the detachment of the external delivery device at time of filling which occurred in 3 cases (3%). The detachment of the thick new balloon, which is partially filled, makes it challenging to puncture with the removal needle and we had to use cautery with difficulty to deflate it prior to removal. All the other complications are expected with intragastric balloons, and the design of our study does not allow us to reach with great certainty in the conclusion that there is a disadvantage with this type of balloon. However, the spontaneous deflation of three balloons one at 6 months and two at 10 months is worrisome and gives a rate of 3% of balloon deflation. Of note is that all three patients were not adherent to their PPI regimen, and this could allow the gastric acid to degrade the balloon faster. It is therefore important in this balloon to stress to the patient the importance of PPI adherence. The increased thickness of the balloon can explain the pancreatitis as it is pushing on the posterior wall of the stomach and exerting a pressure effect on the pancreas without pliability of the wall.
In the eighties, the use of these devices declined due to the high rate of early deflation with associated migration leading to small bowel obstruction. In 1991 and based on the Tarpon Spring Criteria for the ideal gastric balloon, the BioEnterics® intragastric balloon was developed which requires insertion and extraction after 6 months by using endoscopy under sedation [10].
The majority of the endoscopic intragastric balloon literature is on the BioEnterics® Intragastric Balloon (BIB®). A study on the FDA-approved Orbera intragastric balloon system (BIB) that is similar to the Orbers365, approved to stay for 6 months in the stomach, included 321 patients from 8 centers (3 academic, 5 private, 4 surgeons, and 4 gastroenterologists). They reported an early removal rate of 16.6% with only one other complication the form of spontaneous deflation not causing small bowel obstruction. They reported that 8% of patients required IV hydration at an outpatient center. In terms of the effect on weight loss, they found that at 6 months, total body-weight losses of 5%, 10%, and 15% were achieved by 88%, 62%, and 31% of patients [11]. A meta-analysis on the relation of the filling volume and outcome of the BIB balloon reported a pooled %TBWL at 6 months of 13.2% (95% CI 12.3–14.0) [12]. They found no association between the filling volume and %TBWL at 6 months, except when they stratify patients according to the BMI, in which those with a BMI between 40 and 50 kg/m2 had 0.5% TBWL per 100 ml volume. They found no correlation between the filling volume and early removal, gastroesophageal reflux disease (GERD), or ulcers. Higher filling volume was associated with lower rates of esophagitis and migration.
A propensity-matched analysis comparing IGB to laparoscopic bariatric surgery in terms of complications using (MBSAQIP) database found a statistically significant high rate of non-operative intervention associated with IGB placement at 4.2 versus 1.0% in the laparoscopic bariatric surgery cohort. They also found overall adverse outcomes to be significantly higher in the IGB cohort (5.0 versus 2.6%, P = 0.024) [13].
A meta-analysis on BIB safety and effectiveness included 15 studies and 3608 patients found a 12.2% TBWL. Only 13 studies in this meta-analysis which included 3442 patients reported their complications, which were the following: early removal rate was 4.2%, deflation and displacement of the balloon at 2.5%, deflation without displacement of the balloon at 0.9%, obstruction of the digestive tract at 0.9%, gastric ulcer 0.4% and gastric perforation 0.1%, with no pancreatitis was reported in this series [14]. We recently published a case series of pancreatitis with IGB which included BIB, Orbera365, and Spatz. One case in our series was an acute pancreatitis from Orbera365, and this case is different than the two cases included in this cohort, as its not inserted by the authors of this study. All patients had mild pancreatitis and two resolved with conservative management without the need to remove the balloon [15]. In our series of 97 patients who had Orbera365, we report 2 cases of pancreatitis and we removed both balloons due to persistent epigastric pain although the CT scan did not show evidence of severe pancreatitis.
It is thought that IGB should not be left in the stomach for more than 6 months to reduce complications, mainly in the form of ulceration, perforation, deflation, and small bowel obstruction [16]. The first balloon to remain in the stomach for more than 6 months is the Spatz balloon. The pilot study on Spatz examined 18 patients. Seven of the eighteen patients had premature removal of the balloon due to gastritis, valve malfunction, Mallory-Weiss tear, balloon deflation, ulceration, and catheter shear [17]. Genco et al. compared the Spatz to the sequential placement of BIB to achieve a 1-year duration, and found a higher complication rate with Spatz where 7 of 40 patients had complications including deflation in one patient and 4 migrations, where only 2 of 80 patients who had BIB developed complications [10].
In terms of the efficacy of the Orbera365 in weight reduction, at the date of last follow-up, the %TWL was 16.2 ± 10.1, in comparison to 15.5 ± 8.8 6 months post-insertion. This is a good result in terms of weight loss with good maintenance at the time of removal and end of therapy.
Our study contains many limitations including that it is not a multicenter study. We did not study the impact of the Orbera365 device in terms of medical comorbidities nor the quality of life and did not compare it head-to-head with diet only or other noninvasive techniques for weight loss. However, we believe that we present an important information with regard to the safety and effects of the Orbera365 as this is one of the earliest studies on this type of IGB.
Conclusion
In conclusion, the Orbera365 device is effective for weight loss. More studies are needed to determine its safety and in particular to investigate the rate of spontaneous early deflation, device malfunction, tolerance, and pancreatitis.
Data Availability
No data were used to support this study.
Change history
09 December 2021
A Correction to this paper has been published: https://doi.org/10.1007/s11695-021-05827-7
References
Afshin A, Forouzanfar MH, Reitsma MB, et al. Health effects of overweight and obesity in 195 countries over 25 years. N Engl J Med. 2017;377:13–27.
Weiderpass E, Botteri E, Longenecker JC, et al. The prevalence of overweight and obesity in an adult Kuwaiti population in 2014. Front Endocrinol (Lausanne). 2019;10:449.
Dixon JB. Obesity in 2015: advances in managing obesity. Nat Rev Endocrinol. 2016;12(2):65–6.
Barte JC, Terbogt NC, Bogers RP, et al. Maintenance of weight loss after lifestyle interventions for overweight and obesity, a systematic review. Obes Rev. 2010;11(12):899–906.
Khera R, Murad MH, Chandar AK, et al. Association of pharmacological treatments for obesity with weight loss and adverse events: a systematic review and meta-analysis. JAMA. 2016;315(22):2424–34.
Mechanick JI, Apovian C, Brethauer S, et al. Clinical practice guidelines for the perioperative nutrition, metabolic, and nonsurgical support of patients undergoing bariatric procedures - 2019 update: Cosponsored By American Association Of Clinical Endocrinologists/American College Of Endocrinology, The Obesity Society, American Society For Metabolic & Bariatric Surgery, Obesity Medicine Association, And American Society Of Anesthesiologists. Endocr Pract. 2019;25(12):1346–59.
Fuller NR, Pearson S, Lau NS, et al. An intragastric balloon in the treatment of obese individuals with metabolic syndrome: a randomized controlled study. Obesity (Silver Spring). 2013;21(8):1561–70.
Saber AA, Shoar S, Almadani MW, et al. Efficacy of first-time intragastric balloon in weight loss: a systematic review and meta-analysis of randomized controlled trials. Obes Surg. 2017;27(2):277–87.
Imaz I, Martínez-cervell C, García-alvarez EE, et al. Safety and effectiveness of the intragastric balloon for obesity. A meta-analysis Obes Surg. 2008;18(7):841–6.
Genco A, Dellepiane D, Baglio G, et al. Adjustable intragastric balloon vs non-adjustable intragastric balloon: case-control study on complications, tolerance, and efficacy. Obes Surg. 2013;23(7):953–8.
Vargas EJ, Pesta CM, Bali A, et al. Single fluid-filled intragastric balloon safe and effective for inducing weight loss in a real-world population. Clin Gastroenterol Hepatol. 2018;16(7):1073–80.
Kumar N, Bazerbachi F, Rustagi T, et al. The influence of the Orbera intragastric balloon filling volumes on weight loss, tolerability, and adverse events: a systematic review and meta-analysis. Obes Surg. 2017;27:2272–8.
Dang JT, Switzer NJ, Sun WY, et al. Evaluating the safety of intragastric balloon: an analysis of the Metabolic and Bariatric Surgery Accreditation and Quality Improvement Program. Surg Obes Relat Dis. 2018;14(9):1340–7.
Imaz I, Merintez-Carvell C, Garcia-Alvarez EE, et al. Safety and effectiveness of the intragastric balloon for obesity. A Meta-Analysis Obes Surg. 2008;18:841–6.
Alqabandi O, Almutawa Y, AlTarrah D, et al. Intragastric balloon insertion and pancreatitis: case series. Int J Surg Case Rep. 2020;74:263–7.
Machytka E, Klvana P, Kornbluth A, et al. Adjustable intragastric balloons: a 12-month pilot trial in endoscopic weight loss management. Obes Surg. 2011;21:1499–507.
Kannan RY, Nutt MR. Are intra-gastric adjustable balloon system safe? A case series. Int J Surg Case Rep. 2013;4:936–8.
Acknowledgements
The authors are thankful to Carol Dsouza (Senior Research Assistant) for assisting the manuscript.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethics Approval
Ethical approval for conducting the study was obtained from standing committee of Kuwait Ministry of Health (2017/630). Personal information or any information that may lead to identification of patients were not collected. Informed consent was obtained from all subjects included in the study.
Conflict of Interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Key Points
• The Orbera365 is a new balloon that is inserted by endoscopy and can remain in the stomach for 365 days and then is removed by endoscopy.
• The Orbera365 device is effective for weight loss.
• More studies are needed to determine its safety and the rate of spontaneous early deflation, device malfunction, tolerance, and pancreatitis.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Jamal, M.H., Al-Kanawati, N., ElAbd, R. et al. A Study Examining the Orbera365 Intragastric Balloon Safety and Effects on Weight Loss. OBES SURG 31, 5342–5347 (2021). https://doi.org/10.1007/s11695-021-05729-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11695-021-05729-8