Abstract
Background
Following bariatric surgery, accurate charting of weight loss and regain is crucial. Various preoperative factors affect postoperative weight loss, including age, sex, ethnicity, and surgical type. These are not considered by current weight loss metrics, limiting comparison of weight loss outcomes between patients or centers and across time.
Methods
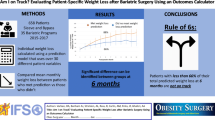
Patients (n=1022) who underwent sleeve gastrectomy (n=809) and gastric bypass (n=213) from 2008 to 2020 in a single center were reviewed. Weight loss outcomes (% total weight loss) were measured for 60 months postoperatively. Longitudinal centile lines were plotted using the post-estimation predictions of quantile regression models, adjusted for type of procedure, sex, ethnicity, and baseline BMI.
Results
Median regression showed that %TWL was 1.0% greater among males than females (β = +1.1, 95% CI: +0.6 to +1.7, P = <0.0001). Participants who underwent SG had less %TWL compared to GB (β = -1.3, 95% CI: -1.9 to -0.8, P < 0.0001). There was a trend towards less %TWL among the Indian and Malay participants compared to Chinese. Age and diabetes were not significant predictors. Reference centile charts were produced for the overall cohort, as well as specific charts adjusted for type of bariatric procedure, sex, ethnicity, and baseline BMI.
Conclusion
Centile charts provide a clinically relevant method for monitoring of weight trajectories postoperatively and aid in realistic and personalised goal setting, and the early identification of “poor responders”. This is the first study to present post-bariatric surgery centile charts for an Asian cohort.



Similar content being viewed by others
References
National Task Force on the Prevention and Treatment of Obesity. Overweight, obesity, and health risk. Arch Intern Med. 2000;160(7):898. https://doi.org/10.1001/archinte.160.7.898.
Sjöström L. Review of the key results from the Swedish Obese Subjects (SOS) trial - a prospective controlled intervention study of bariatric surgery. J Intern Med. 2013;273(3):219–34. https://doi.org/10.1111/joim.12012.
Lee PC, Tham KW, Ganguly S, et al. Ethnicity does not influence glycemic outcomes or diabetes remission after sleeve gastrectomy or gastric bypass in a multiethnic Asian cohort. Obes Surg. 2018;28(6):1511–8. https://doi.org/10.1007/s11695-017-3050-6.
Syn NL, Cummings DE, Wang LZ, et al. Association of metabolic–bariatric surgery with long-term survival in adults with and without diabetes: a one-stage meta-analysis of matched cohort and prospective controlled studies with 174 772 participants. Lancet. 2021;397(10287):1830–41. https://doi.org/10.1016/S0140-6736(21)00591-2.
Bray GA, Heisel WE, Afshin A, et al. The science of obesity management: an endocrine society scientific statement. Endocr Rev. 2018;39(2):79–132. https://doi.org/10.1210/er.2017-00253.
Hatoum IJ, Stein HK, Merrifield BF, et al. Capacity for physical activity predicts weight loss after roux-en-y gastric bypass. Obesity. 2009;17(1):92–9. https://doi.org/10.1038/oby.2008.507.
Contreras JE, Santander C, Court I, et al. Correlation between age and weight loss after bariatric surgery. Obes Surg. 2013;23(8):1286–9. https://doi.org/10.1007/s11695-013-0905-3.
Perrone F, Bianciardi E, Benavoli D, et al. Gender influence on long-term weight loss and comorbidities after laparoscopic sleeve gastrectomy and roux-en-y gastric bypass: a prospective study with a 5-year follow-up. Obes Surg. 2016;26(2):276–81. https://doi.org/10.1007/s11695-015-1746-z.
Lager CJ, Esfandiari NH, Subauste AR, et al. roux-en-y gastric bypass vs. sleeve gastrectomy: balancing the risks of surgery with the benefits of weight loss. Obes Surg. 2017;27(1):154–61. https://doi.org/10.1007/s11695-016-2265-2.
Li J-F, Lai D-D, Ni B, et al. Comparison of laparoscopic Roux-en-Y gastric bypass with laparoscopic sleeve gastrectomy for morbid obesity or type 2 diabetes mellitus: a meta-analysis of randomized controlled trials. Can J Surg. 2013;56(6):E158–64. https://doi.org/10.1503/cjs.026912.
van de Laar AW, Nienhuijs SW, Apers JA, et al. The Dutch bariatric weight loss chart: A multicenter tool to assess weight outcome up to 7 years after sleeve gastrectomy and laparoscopic Roux-en-Y gastric bypass. Surg Obes Relat Dis. 2019;15(2):200–10. https://doi.org/10.1016/j.soard.2018.11.024.
Manning S, Pucci A, Carter NC, et al. Early postoperative weight loss predicts maximal weight loss after sleeve gastrectomy and Roux-en-Y gastric bypass. Surg Endosc. 2015;29(6):1484–91. https://doi.org/10.1007/s00464-014-3829-7.
WHO Expert Consultation. Appropriate body-mass index for Asian populations and its implications for policy and intervention strategies. Lancet. 2004;363(9403):157–63. https://doi.org/10.1016/S0140-6736(03)15268-3.
Rubino F, Nathan DM, Eckel RH, et al. Metabolic surgery in the treatment algorithm for type 2 diabetes: a joint statement by international diabetes organizations. Diabetes Care. 2016;39(6):861–77. https://doi.org/10.2337/dc16-0236.
Coleman KJ, Huang Y-C, Hendee F, et al. Three-year weight outcomes from a bariatric surgery registry in a large integrated healthcare system. Surg Obes Relat Dis. 2014;10(3):396–403. https://doi.org/10.1016/j.soard.2014.02.044.
Bray GA, Bouchard C, Church TS, et al. Is it time to change the way we report and discuss weight loss? Obesity. 2009;17(4):619–21. https://doi.org/10.1038/oby.2008.597.
Lee YS, Biddle S, Chan MF, et al. Health Promotion Board-Ministry of Health Clinical Practice Guidelines: Obesity. Singapore Med J. 2016;57(6):292–300. https://doi.org/10.11622/smedj.2016103.
Lim CH, Lee PC, Lim E, et al. Correlation between symptomatic Gastro-Esophageal Reflux Disease (GERD) and Erosive Esophagitis (EE) Post-vertical sleeve gastrectomy (VSG). Obes Surg. 2019;29(1):207–14. https://doi.org/10.1007/s11695-018-3509-0.
Lim CH, Jahansouz C, Abraham AA, et al. The future of the Roux-en-Y gastric bypass. Expert Rev Gastroenterol Hepatol. 2016;10(7):777–84. https://doi.org/10.1586/17474124.2016.1169921.
Chew CAZ, Tan IJ-W, Ng HJH, et al. Early weight loss after laparoscopic sleeve gastrectomy predicts midterm weight loss in morbidly obese Asians. Surg Obes Relat Dis Off J Am Soc Bariatr Surg. 2017;13(12):1966–72. https://doi.org/10.1016/j.soard.2017.05.016.
Lee PC, Dixon JB, Sim PY, et al. Treatment options for poor responders to bariatric surgery. Curr Obes Rep. 2020;9(3):364–72. https://doi.org/10.1007/s13679-020-00381-2.
Ogden J, Clementi C, Aylwin S. The impact of obesity surgery and the paradox of control: A qualitative study. Psychol Health. 2006;21(2):273–93. https://doi.org/10.1080/14768320500129064.
Zhang Y, Zhao H, Cao Z, et al. A randomized clinical trial of laparoscopic roux-en-y gastric bypass and sleeve gastrectomy for the treatment of morbid obesity in China: a 5-year outcome. Obes Surg. 2014;24(10):1617–24. https://doi.org/10.1007/s11695-014-1258-2.
Ng J, Seip R, Stone A, et al. Ethnic variation in weight loss, but not co-morbidity remission, after laparoscopic gastric banding and Roux-en-Y gastric bypass. Surg Obes Relat Dis. 2015;11(1):94–100. https://doi.org/10.1016/j.soard.2014.07.013.
Wee CC, Jones DB, Apovian C, et al. Weight loss after bariatric surgery: do clinical and behavioral factors explain racial differences? Obes Surg. 2017;27(11):2873–84. https://doi.org/10.1007/s11695-017-2701-y.
Koh ZJ, Tai BC, Kow L, et al. Influence of Asian ethnicities on short- and mid-term outcomes following laparoscopic sleeve gastrectomy. Obes Surg. 2019;29(6):1781–8. https://doi.org/10.1007/s11695-019-03716-8.
Campos GM. Factors associated with weight loss after gastric bypass. Arch Surg. 2008;143(9):877–883; discussion 884. https://doi.org/10.1001/archsurg.143.9.877.
Luo Y, Eldin AWJ, Haddad R, et al. MON-590 presence of diabetes diminishes the ultimate weight loss after bariatric surgery. J Endocr Soc. 2020;4(Supplement_1):MON-590. https://doi.org/10.1210/jendso/bvaa046.813.
Cazzo E, da Silva FP, Pareja JC, et al. Predictors for weight loss failure following roux-en-y gastric bypass. Arq Gastroenterol. 2014;51(4):328–30. https://doi.org/10.1590/S0004-28032014000400011.
Wei Y, Pere A, Koenker R, et al. Quantile regression methods for reference growth charts. Stat Med. 2006;25(8):1369–82. https://doi.org/10.1002/sim.2271.
Kiserud T, Piaggio G, Carroli G, et al. The World Health Organization Fetal Growth Charts: A Multinational Longitudinal Study of Ultrasound Biometric Measurements and Estimated Fetal Weight. PLoS Med. 2017;14(1):e1002220. https://doi.org/10.1371/journal.pmed.1002220
Kiserud T, Benachi A, Hecher K, et al. The World Health Organization fetal growth charts: concept, findings, interpretation, and application. Am J Obstet Gynecol. 2018;218(2):S619–29. https://doi.org/10.1016/j.ajog.2017.12.010.
Stifel DC, Averett SL. Childhood overweight in the United States: A quantile regression approach. Econ Hum Biol. 2009;7(3):387–97. https://doi.org/10.1016/j.ehb.2009.05.005.
Ochner CN, Jochner MCE, Caruso EA, et al. Effect of preoperative body mass index on weight loss after obesity surgery. Surg Obes Relat Dis. 2013;9(3):423–7. https://doi.org/10.1016/j.soard.2012.12.009.
Author information
Authors and Affiliations
Contributions
Concept and design: NL Syn, PC Lee, CH Lim
Acquisition, analysis, or interpretation of data: NL Syn, SYT Tan, D Lin, PC Lee, S Ganguly, CH Lim
Interpretation of data: NL Syn, SYT Tan, D Lin, PC Lee, S Ganguly, CH Lim
Drafting of the manuscript: NL Syn, SYT Tan, D Lin, PC Lee, S Ganguly, CH Lim
Critical revision of the manuscript for important intellectual content: NL Syn, SYT Tan, D Lin, PC Lee, S Ganguly, Ong HS, J Tan, CH Lim
Study supervision: NL Syn, SYT Tan, D Lin, PC Lee, CH Lim
Corresponding author
Ethics declarations
Ethics Approval and Consent to Participate
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed consent was obtained from all individual participants included in the study.
Conflict of Interest
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Key Points
• Centile charts provide a clinically relevant method to monitor weight trajectories after bariatric surgery
• The use of procedure, sex, ethnicity and BMI-specific charts allow more accurate monitoring and personalised goal-setting
• Following validation, these charts can be used in both clinical and research settings
Rights and permissions
About this article
Cite this article
Tan, S.Y.T., Syn, N.L., Lin, D.J. et al. Centile Charts for Monitoring of Weight Loss Trajectories After Bariatric Surgery in Asian Patients. OBES SURG 31, 4781–4789 (2021). https://doi.org/10.1007/s11695-021-05618-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11695-021-05618-0