Abstract
Obesity in the young is increasingly prevalent. Early, effective intervention is paramount. Treatment options are lifestyle modifications, pharmacological therapies, endoscopic treatments and bariatric surgery. However, the relative effectiveness of these treatments in young patients remains unclear. We systematically identify and meta-analyse studies evaluating weight loss treatments in young people (< 21 years) with obesity. From 16,372 identified studies, 83 were eligible for meta-analysis. Bariatric surgery resulted in high short/medium-term weight loss (pooled estimate 14.04 kg/m2). Lifestyle and pharmacological therapies impacted weight more moderately (pooled estimate 0.99 and 0.94 kg/m2 respectively). Due to its high efficacy, bariatric surgery should be considered earlier when treating obesity in young people. However, due to the invasiveness and inherent risks of bariatric surgery, all other weight loss routes should be exhausted first.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Obesity has a high and increasing prevalence in the young population [1]. The National Child Measurement Programme in England 2015–2016 found that 19.8% of children aged 10 to 11 years are currently obese [2]. Similarly, the National and Nutrition Examination Survey carried out in USA found that 20.5% of adolescents aged 12 to 19 are obese [3]. Importantly, childhood obesity is associated with a 16 times increased risk of adult obesity compared to normal-weight children [4]. Moreover, obesity results in an increased risk of type 2 diabetes, hypertension, dyslipidaemia, cardiovascular diseases, cancer, psychological conditions and mortality [5]. Hence, there is a critical unmet need for innovative therapeutic strategies that address the treatment of obesity in young people. Early intervention in this cohort is likely to bring added benefits by preventing the cumulative deleterious health effects of obesity-related chronic disease and, in doing so, will reduce the associated cost burden [5].
Current treatment options for obesity include lifestyle modifications, pharmacological therapies, endoscopic treatments and bariatric surgery [6]. Bariatric surgery is currently the gold standard treatment for long-term weight loss in adults [7]. However, the optimal treatment for young people is not known. The causes of obesity in young people as well as their biological and psychological response to interventions differ from those of adults. Younger patients are less habitualised and may be more easily persuaded to undergo long-term lifestyle changes compared to their elder counterparts. Alternatively, children may be less compliant at taking weight loss medication on a daily basis.
The most recent review examining weight loss in young people dates from 2008 [6]. However, this review only evaluated lifestyle modifications and pharmacological therapies [6]. Since this, there have been a number of new trials that have added weight to this body of evidence.
The present systematic review and meta-analysis aims to be the first study to comprehensively summarise and quantify the comparative efficacy of body mass index (BMI)-reducing treatment options in the young population with obesity. It will help clinicians determine suitable courses of treatment in this cohort in order to more successfully achieve weight loss and, subsequently, improve associated comorbidities—an important issue in the ever-growing epidemic.
Methods
The systematic review and meta-analysis was conducted according to the principles endorsed in the PRISMA-P statement [8].
Literature Search Strategy
An extensive literature search of the MEDLINE and EMBASE databases from inception (last search May 2016) was conducted. Search terms were included to detect studies investigating lifestyle modifications, pharmacological therapies, endoscopic treatments and bariatric surgery in the young population with obesity (Supplementary Appendix 1).
Eligibility Criteria
Studies were selected according to the following pre-defined eligibility criteria. Type of participant: We define young people as those aged 21 years or younger. Thus, included study participants were aged 21 years or younger with a BMI of 25 kg/m2 or higher. Studies with pregnant females, neonates and patients with obesity-related genetic syndromes (e.g. Prader Willi syndrome) were excluded. Type of intervention: Four interventions were included: lifestyle modifications, pharmacological therapies, endoscopic treatments and bariatric surgery. There was no restriction on who delivered the interventions, e.g. specialist doctors or teachers. The interventions could be community-, school- or clinic-based. Type of comparison: The highest level of available evidence was used in each group. Lifestyle and drug studies were all randomised control trials and had controls which had either no intervention, placebos or minimal interventions such as a single information session. Only a single randomised control trial (RCT) was retrieved assessing surgical management, so non-randomised studies were included for this sub-analysis. Type of outcome measure: Studies included one or more of the following outcomes: weight (kg), BMI (kg/m2), BMI standard deviation score (BMI-SDS or BMI-Z score), waist circumference (cm), fat mass (kg) or waist to hip ratio. These could either be reported as a primary or secondary outcome of the study.
Two independent reviewers (SS and NA) assessed the search strategy results. Study titles were examined for potential relevance and the abstract was then reviewed. Subsequently, the full text was retrieved to ensure eligibility. Review articles and studies with insufficient data for meta-analysis were excluded. No limits were applied for language and foreign papers. This involved the translation of one Portuguese study [9]. The bibliographies of relevant articles were inspected for further eligible studies. In the case of any uncertainty regarding study inclusion, another investigator (NP) was consulted to assess eligibility.
Data Extraction
Eligible studies were sub-divided into four groups: lifestyle modifications, pharmacological therapies, endoscopic treatments and bariatric surgery. For the included studies, the following variables were obtained: study title, publication year, first author, type of intervention, number of participants, metric of measure for weight loss, baseline control and interventional group weight metrics, post-intervention control and interventional group weight metrics. Due to a sparsity of evidence using other weight metrics, BMI was utilised for the quantitative analysis.
Assessment of Methodological Quality
Methodological quality of RCTs was assessed using the Cochrane Collaboration Tool (Supplementary Appendix 2) [10]. This included assessment of random sequence generation, allocation concealment, blinding of participants and assessors, incomplete outcome data, selective reporting and other sources of bias [10].
Statistical Analysis
Any missing standard deviations were derived from other metrics provided, using standard formula found in the Cochrane handbook. The chi-squared test was completed to establish the degree of heterogeneity [11]. Higgins et al. suggest that 25, 50 and 75% indicate low, medium and high heterogeneity respectively [11]. As I2 values were over 75%, the restricted maximum likelihood random-effects method was used to generate pooled mean BMI differences [11]. Publication bias was assessed using funnel plots (Supplementary Appendix 3). Statistical analyses were performed using the Metafor package in R (version 3.2.4).
Results
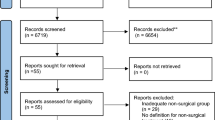
The comprehensive literature search identified 16,372 studies. After de-duplication and eligibility exclusions, 93 studies were included in the systematic review (Fig. 1). Eighty-three studies underwent quantitative analysis: 29 for lifestyle intervention (Fig. 2), 21 for pharmacological therapies (Fig. 3) and 33 for bariatric surgery (Fig. 4). Ten studies assessing endoscopic treatment were included for qualitative analysis. Study characteristics, patient characteristics, intervention description, side effects/complications and adherence are described for lifestyle intervention, pharmacological therapies, endoscopic treatments and bariatric surgery (Table 1) respectively. A detailed description of the complications associated with bariatric surgery within the included studies is provided in Table 2. A list of the included studies is provided in Supplementary Appendix 4.
Lifestyle Modifications
Behaviour
Analysis revealed behavioural interventions decreased mean BMI by 0.18 kg/m2 (95%CI − 0.52 to 0.15). One study continued for longer than 12 months [12]. Taylor et al. illustrated that monthly advice sessions and motivational interviews led to a statistically significant mean BMI reduction of 0.34 kg/m2 in overweight/obese 4- to 8-year-old patients at 24 months [12].
Exercise
Pooled analysis demonstrated that mean BMI reduced by 0.89 kg/m2 (95%CI − 2.20 to 0.42), compared to controls. One study continued for longer than 6 months [13]. Kokkvoll et al. found that over a 2-year intervention, patients continued to gain weight despite exercise [13].
Diet
Dietary interventions were trialled in three studies [14,15,16]. Pooled analysis revealed a reduction of mean BMI by 0.91 kg/m2 (95%CI − 1.80 to − 0.02) compared with comparison groups. Okeley et al. and Shalitin et al. found that diet was significantly better at reducing BMI than exercise at 12 months [14, 15]. However, Shalitin et al. discovered that patients regained any lost weight 9 months after suspension of intervention [14]. Ebbling et al. found that a low glycaemic index diet caused a statistically significant reduction in BMI compared to a low-fat diet [16]. None of the studies were carried out for longer than 12 months.
Behaviour, Exercise and Diet
Pooled analysis revealed that combined lifestyle intervention reduced mean BMI by 1.34 kg/m2 (95%CI − 2.19 to − 0.50) at 6 months and by 1.52 kg/m2 (95%CI − 2.97 to − 0.07) at 12 months, revealing a non-significant difference between the two time intervals. A combined lifestyle approach illustrated an overall reduction in mean BMI of 1.44 kg/m2 (95%CI − 2.23 to − 0.65. This proved to be more effective than a single lifestyle intervention of either diet, exercise or behaviour. One study was carried out for longer than 12 months [17]. Kalarchian et al. found that the majority of 8- to 12-year-old patients regained their lost weight at 18 months [17].
The sub-group meta-analysis at 12 months of combined lifestyle interventions contains an outlier. Weigel et al. illustrated a much greater BMI reduction compared to other studies [18]. It consisted of intensive interventions with bi-weekly sessions for 12 months [18]. Other studies reduced their intervention frequency as the study progressed. However, after removing the outlier and re-conducting the meta-analysis, the results still remained statistically significant with a reduction of BMI by 1.06 kg/m2 (95%CI − 1.78 to − 0.35) at 6 months and 0.86 kg/m2 (95%CI − 1.61 to − 0.10) at 12 months.
Pharmacological Therapies
Metformin
Pooled analysis revealed metformin reduced mean BMI by 0.75 kg/m2 (95%CI − 1.14 to − 0.35) compared with the placebo. There was a statistically insignificant difference between studies that prescribed metformin at different time intervals. Two of the 14 studies were continued for longer than 6 months [19, 20]. Nwosu et al. found a statistically insignificant difference in BMI between baseline and study conclusion in the interventional group [19]. Wilson et al. illustrated that the treatment group had a higher BMI than the control group at 18 months due to rapid weight gain after metformin was withdrawn [20].
Sibutramine
Pooled analysis for sibutramine demonstrated a decrease in mean BMI of 1.66 kg/m2 (95%CI − 2.48 to − 0.84) compared to the placebo. None of the studies evaluated BMI change maintenance.
Orlistat
Analysis revealed that orlistat decreased mean BMI by 0.87 kg/m2 (95%CI − 1.19 to − 0.54) compared to the placebo. Two studies were longer than 6 months [21, 22]. Chanoine et al. illustrated a reduction in mean BMI of 0.55 kg/m2 in the interventional group at 12 months [21]. Ozkan et al. found similar results after 11.7 months [22].
Endoscopic Treatments
Intragastric Balloon
Only one study reported a statistically insignificant decrease in percentage change of BMI after 3 months (p = 0.07) [23]. At 6 months, these patients regained any lost weight and became more obese than before intragastric balloon (IGB) insertion [23]. Two studies mentioned weight loss maintenance [24, 25]. Adorisio et al. demonstrated that 24 months after balloon removal, 48.4% of patients maintained/continued weight loss in 11- to 21-year-old patients [25]. Curran et al. revealed that any weight loss achieved by lifestyle or IGB interventions was not maintained at 18 months [24].
Endobarrier
One conference abstract evaluated the Endobarrier [26]. At 6 months after insertion, all patients experienced significant weight loss with a mean weight reduction of 20.8% [26].
Bariatric Surgery
Laparoscopic Adjustable Banding
Pooled analysis revealed that laparoscopic adjustable banding (LAGB) decreased mean BMI by 9.74 kg/m2 (95%CI − 11.61 to − 7.87) at 12 months post surgery. Nine studies were longer than 12 months [27,28,29,30,31,32,33,34]. All of these studies showed significant and sustained BMI reduction in young patients over a longer follow-up period [27,28,29,30,31,32,33,34]. These longer studies demonstrated an excess weight loss between 45 and 57.5% at study conclusion [27,28,29,30,31,32,33,34]. LAGB demonstrated a relatively good safety profile in the paediatric population with the most common complication being asymptomatic iron deficiency. However, one death was reported at 46 months following acute haemorrhage after band removal [35].
Roux-en-Y Gastric Bypass
Pooled analysis showed Roux-en-Y gastric bypass (RYGB) decreased BMI by 17.06 kg/m2 (95%CI − 19.68 to − 14.45) at 12 months post surgery. Three trials continued for longer than 12 months [36,37,38]. Olbers et al. stated that there were a statistically insignificant change in BMI between 12 and 24 months in the surgical group [37]. Teeple et al. and Tsamis et al. revealed an excess weight loss of more than 50% at 24 months. Reduction in weight was maintained at 48 months [36, 38]. The most common complication following RYGB was vitamin and/or iron deficiency.
Laparoscopic Sleeve Gastrectomy
Pooled analysis revealed laparoscopic sleeve gastrectomy (LSG) decreased mean BMI by 16.13 kg/m2 (95%CI − 17.92 to − 14.33). Four studies were carried out for longer than 12 months [39,40,41,42]. Patients maintained any weight loss achieved in the short term [39,40,41,42]. At study conclusion, which varied between 24 and 60 months across trials, all patients decreased their BMI by more than 18 kg/m2 [39,40,41,42]. The most common complication following LSG was gastroesophageal reflux. Importantly, evidence regarding the long-term complications of bariatric surgery in young patients is still sparse and should not be underestimated.
Discussion
Key Statistics
Lifestyle BMI (kg/m2) mean difference | − 0.99 (95%CI − 1.43 to − 0.53) |
Drugs BMI (kg/m2) mean difference | − 0.94 (95%CI − 1.30 to − 0.59) |
Surgery BMI (kg/m2) mean difference | − 14.04 (95%CI − 15.65 to − 12.43) |
Principle Findings
This study investigated the comparative efficacy of BMI-reducing treatment options for obesity in the young population. It illustrates that as a single intervention, bariatric surgery is the most effective treatment for BMI reduction in the short and long terms. Furthermore, LSG and RYGB caused a statically significant reduction in BMI compared to LAGB at 12 months.
Although this systematic review and meta-analysis is not designed to evaluate the relative risks between different treatment modalities for obesity in young patients, it is important that the risks that accompany surgery are well established. This study suggests that bariatric surgery has a good safety profile in the paediatric population. However, there are existing concerns over the long-term consequences of bariatric surgery on pre-pubertal children with severe obesity, including factors such as growth. Recently, Alqahtani et al. showed that, contrary to expectations, growth was improved after LSG, with approximately 1 extra millimetre of height gain per month compared with obese children who did not undergo surgery [41]. However, of the 2155 patients included that underwent surgical intervention, one death relating to the surgery was reported 48 months following LAGB [35]. The 19-year-old patient underwent emergency endoscopic evaluation 4 years post-gastric banding due to melena and haematemesis. Gastric haemorrhage with band erosion was found. During gastric band removal, acute haemorrhage occurred and the patient subsequently died of uncontrolled bleeding [35]. This illustrates that although rare, severe post-operative complications are possible. Furthermore, evidence regarding the long-term complications associated with bariatric surgery on young patients is still sparse [43]. Therefore, it is important that the risks that accompany surgery are not underestimated and are well established in each individual case [44].
Moreover, evidence regarding the long-term efficacy of bariatric surgery in young patients is limited. However, recently, Olbers et al. conducted a prospective study analysing 5-year outcomes of adolescent patients after Roux-en-Y gastric bypass. It demonstrated a BMI reduction of 13.5 kg/m2 compared to a control group undergoing lifestyle intervention that experienced an increase in BMI of 3.3 kg/m2 [45]. Additionally, Inge et al. analysed 3-year outcomes of adolescent patients after Roux-en-Y gastric bypass and sleeve gastrectomy. The study found that patient BMI decreased by 15 and 13 kg/m2, respectively [46]. Nevertheless, despite emerging studies that demonstrate the longer-term efficacy of bariatric surgery in adolescent patients, it is important to highlight that long-term data in this cohort is still limited and further studies are required.
Assessment of lifestyle interventions revealed that a combined approach of behaviour, exercise and dietary treatments resulted in a statistically significant greater BMI reduction compared to a solitary lifestyle intervention. Moreover, both lifestyle modifications and pharmacological therapies have similar impacts on BMI change in the short and medium terms.
Sibutramine appeared to be the most effective drug at achieving BMI loss in the short term. It facilitates weight loss by acting as a serotonin reuptake inhibitor causing activation of anorexigenic pathways to prevent cognitive sensations of hunger [47].
Metformin caused similar BMI changes over short- and medium-term administration periods. However, there is no clear benefit on BMI loss after long-term metformin administration compared with short- and medium-term. The mechanism by which metformin results in weight loss is unclear, but it is likely to be multifactorial [48]. Its effect on weight may be mediated by increasing glucagon-like peptide 1, reducing dipeptidyl peptidase-4 activity, modifying the gut microbiome, increasing lipid oxidation and promoting central satiety [48].
Orlistat inhibits gastric and pancreatic lipase and decreased absorption of triglycerides by approximately 30% [49]. It is the only weight-reducing drug approved for paediatric patients with obesity in USA [49]. However, it is imperative that more studies investigate the weight-reducing effects of orlistat in young adults to conclusively determine whether it should be preferred to other available treatment options.
Endoscopic treatments appear to cause statistically significant weight loss in the short term. However, given the low number of studies included, it is vital that more studies are conducted to further assess its impact on weight loss. The mechanism behind the IGB includes the inflation of a balloon in the stomach of patients to fill its capacity, promoting satiety and decreasing calorie intake [50]. The Endobarrier leads to weight loss by creating a 60-cm-length barrier along the duodenal wall to reduce absorption of nutrients [51].
Strengths and Limitations
To our knowledge, this is the first comprehensive systematic review and meta-analysis to directly compare the efficacy of weight-reducing treatment options for obesity in the young population. Lifestyle modification and pharmacological therapy meta-analyses contain a large number of RCTs. The surgical meta-analysis contains five times the number of overall patients and double the number of LSG studies compared to the most recent topical paper [52].
This study includes sibutramine despite its withdrawal from the USA and UK market following the SCOUT study [53]. It was illustrated that sibutramine carries a relative risk of 1.16 for causing cardiac-related morbidity [54]. However, sibutramine can be found in small amounts within some diet pills and herbal remedies, which can be purchased online [55]. Therefore, it is important to know its possible impact on young people as well as using it as a historical benchmark to compare against current licenced medications.
Surprisingly, dietary interventions were rarely evaluated as a sole component of treatment in comparison with a minimal-intervention or no-treatment control group [56]. Therefore, many trials did not meet the inclusion criteria for statistical analysis in this study. Nevertheless, the available evidence demonstrates that dietary interventions do have the potential to cause a statistically significant decrease in BMI. Partsalaki et al. compared the efficacy of a ketogenic and a hypocaloric diet in obese children without the use of a control group [57]. At 6 months, both groups had a statistically significant decline in mean BMI of 3.7 and 3.3 kg/m2, respectively. This illustrates a higher BMI reduction compared to RCTs included in this study. There is a clear need for well-designed RCTs that assess the long-term efficacy of alternative dietary interventions to allow conclusive determinations regarding its efficacy.
There is a lack of information from RCTs on sibutramine and its impact on BMI change after 6 months. A prospective observational study demonstrated that a large dropout rate was seen due to a slow rate of weight reduction after 6 months compared to the first 6 months of administration [47]. It was concluded that sibutramine has a limited effect on weight loss in the long term [47].
The surgical meta-analysis included non-randomised trials (Fig. 4) making it difficult to directly compare the efficacy of surgery to other interventions. The studies do not take into account the placebo effect and allow bias from potential confounding factors [58]. However, the lack of RCTs reflects the ethical concerns of randomising a vulnerable population, in this case children and adolescents with obesity [52]. Surgery includes a higher risk of complications and a greater cost, making it difficult to carry out RCTs in this area [59].
Another limitation includes the use of BMI to measure weight change in a paediatric population [59]. BMI can be inaccurate, as the calculation does not distinguish between lean and fat mass [59]. Moreover, variation in age, sex and maturation results in a large range of normal BMI scores in the paediatric population [59]. BMI-Z score would be a better measure of weight change as it considers age and gender [59]. However, studies most commonly reported BMI, and therefore, it was the used metric for this analysis.
The results from this meta-analysis apply to the general paediatric population of overweight and obese individuals below the age of 21 with a BMI of 25 kg/m2 or more. In the included studies, mean age ranged from 4.8 to 19 years. As a whole, the patients undergoing bariatric surgery were older than those carrying out lifestyle modifications. This may have influenced findings. However, the limited number of studies prevented investigations through further stratification of results according to age groups.
Finally, a lack of studies with large sample sizes is a problem when evaluating the efficacy of orlistat and endoscopic treatment. It is important that more studies are carried out to further assess their impact on weight loss.
Comparison with Other Studies
McGovern et al. (2006) concluded that a combined lifestyle intervention is more effective than a single lifestyle intervention approach on BMI change and that pharmacological therapy (for example, sibutramine) can be beneficial in the short term [60]. Our study confirms this. We also extend this inference to note that overall lifestyle and pharmacological interventions have very similar impacts on BMI reduction, while metformin demonstrates similar effects on BMI reduction regardless of the time interval for which it is administered. Our results are also consistent with another review that found that metformin did not demonstrate any clear benefit on weight loss after long-term administration [61].
Our surgical meta-analysis contained solely non-randomised trials; however, its results mirrored the only published RCT in this area [62]. This RCT of 50 patients illustrated that the surgical arm reduced mean BMI by 12.7 kg/m2 (95%CI − 11.3 to − 14.2), compared to a reduction of 1.3 kg/m2 (95%CI − 0.4 to − 2.9) in the lifestyle modification control arm [62].
Conclusion
This meta-analysis conclusively determines the comparative efficacy of weight-reducing treatments in obese young patients. Currently, bariatric surgery is rarely considered in this cohort. However, this meta-analysis provides comprehensive evidence that compared with non-surgical obesity treatments, bariatric surgery leads to a greater BMI reduction in the short and long terms. Therefore, this data suggests that physicians and patients should have a lower threshold for considering bariatric surgery when lifestyle and pharmacological interventions have failed. Nevertheless, there should be a clear understanding of the risks this treatment option may entail for each individual young patient. Furthermore, future RCTs are required to enable a conclusive determination regarding long-term risks of bariatric surgery in young people. Due to the stigma and psychological impact of obesity, it is important that physicians approach this topic sensitively. There should be effective communication discussing the relative efficacy of all treatment options and their associated complications between those involved. The evidence for certain treatment options, in particular endoscopic treatments, remains unclear and calls for further research. This knowledge will assist physicians in determining a holistic, patient-centred treatment programme for young patients with obesity in order to achieve successful BMI reduction and subsequent improvement of associated comorbidities.
Change history
07 June 2018
In the original article, due to a production error, the text for the “Principle Findings” section was omitted as was the heading for the “Strengths and Limitations” section. The original article has been updated to correct these errors.
References
Han JC, Lawlor DA, Kimm SY. Childhood obesity. Lancet. 2010;375(9727):1737–48.
Craig R, Mindell J, Boodhna G. Health survey for England, 2013. 2014.
Ogden CL, Carroll MD, Fryar CD, Flegal KM. Prevalence of obesity among adults and youth: United States, 2011–2014. NCHS Data Brief 2015;(219):1–8.
Suchindran C, North KE, Popkin BM, et al. Association of adolescent obesity with risk of severe obesity in adulthood. JAMA. 2010;304(18):2042–7.
Welbourn R, le Roux CW, Owen-Smith A, et al. Why the NHS should do more bariatric surgery; how much should we do? BMJ. 2016;353:i1472.
Oude Luttikhuis H, Baur L, Jansen H, et al. Interventions for treating obesity in children. Cochrane Database Syst Rev. 2009;1(1)
Schauer PR, Kashyap SR, Wolski K, et al. Bariatric surgery versus intensive medical therapy in obese patients with diabetes. N Engl J Med. 2012;366(17):1567–76.
Moher D, Shamseer L, Clarke M, et al. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015 statement. System Rev. 2015;4(1):1.
Alves JGB, Galé CR, Souza E, et al. Effect of physical exercise on bodyweight in overweight children: a randomized controlled trial in a Brazilian slum. Cadernos de Saúde Pública. 2008;24:s353–9.
Higgins JP, Altman DG, Gotzsche PC, et al. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ. 2011;343:d5928.
Higgins J, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med. 2002;21(11):1539–58.
Taylor RW, Cox A, Knight L, et al. A tailored family-based obesity intervention: a randomized trial. Pediatrics. 2015;136(2):281–9.
Kokkvoll A, Grimsgaard S, Steinsbekk S, et al. Health in overweight children: 2-year follow-up of Finnmark Activity School—a randomised trial. Arch Dis Child. 2015;100(5):441–8.
Shalitin S, Ashkenazi-Hoffnung L, Yackobovitch-Gavan M, et al. Effects of a twelve-week randomized intervention of exercise and/or diet on weight loss and weight maintenance, and other metabolic parameters in obese preadolescent children. Horm Res. 2009;72(5):287–301.
Okely AD, Collins CE, Morgan PJ, et al. Multi-site randomized controlled trial of a child-centered physical activity program, a parent-centered dietary-modification program, or both in overweight children: the HIKCUPS study. J Pediatr. 2010;157(3):388–94. e1
Ebbeling CB, Leidig MM, Sinclair KB, et al. A reduced–glycemic load diet in the treatment of adolescent obesity. Arch Pediatr Adolesc Med. 2003;157(8):773–9.
Kalarchian MA, Levine MD, Arslanian SA, et al. Family-based treatment of severe pediatric obesity: randomized, controlled trial. Pediatrics. 2009;124(4):1060–8.
Weigel C, Kokocinski K, Lederer P, et al. Childhood obesity: concept, feasibility, and interim results of a local group-based, long-term treatment program. J Nutr Educ Behav. 2008;40(6):369–73.
Nwosu BU, Maranda L, Cullen K, et al. A randomized, double-blind, placebo-controlled trial of adjunctive metformin therapy in overweight/obese youth with type 1 diabetes. PLoS One. 2015;10(9):e0137525.
Wilson DM, Abrams SH, Aye T, et al. Metformin extended release treatment of adolescent obesity: a 48-week randomized, double-blind, placebo-controlled trial with 48-week follow-up. Arch Pediatr Adolesc Med. 2010;164(2):116–23.
Chanoine J, Hampl S, Jensen C, et al. Effect of orlistat on weight and body composition in obese adolescents: a randomized controlled trial. JAMA. 2005;293(23):2873–83.
Ozkan B, Bereket A, Turan S, et al. Addition of orlistat to conventional treatment in adolescents with severe obesity. Eur J Pediatr. 2004;163(12):738–41.
Vandenplas Y, Bollen P, De Langhe K, et al. Intragastric balloons in adolescents with morbid obesity. Eur J Gastroenterol Hepatol. 1999;11(3):243–5.
Curran J, Kalic R, Sherrington C, et al. RCT of intragastric balloons in adolescents: preliminary data. Obes Res Clin Pract. 2011;5:24–5.
De Peppo F, Adorisio O, Melissa B, et al. BioEnterics Intragastric Balloon for the treatment of pathologic obesity in Prader–Willi patients. Paediatr Child Health. 2009;19:S38–42.
Kotnik P, Homan M, Battelino T. Initial experience with endoscopically placed duodenal-jejunal bypass liner (Endobarrier) in morbidly obese adolescents. 2015.
Holterman A, Browne A, Tussing L, et al. A prospective trial for laparoscopic adjustable gastric banding in morbidly obese adolescents: an interim report of weight loss, metabolic and quality of life outcomes. J Pediatr Surg. 2010;45(1):74–9.
Nadler EP, Youn HA, Ginsburg HB, et al. Short-term results in 53 US obese pediatric patients treated with laparoscopic adjustable gastric banding. J Pediatr Surg. 2007;42(1):137–42.
Nadler EP, Youn HA, Ren CJ, et al. An update on 73 US obese pediatric patients treated with laparoscopic adjustable gastric banding: comorbidity resolution and compliance data. J Pediatr Surg. 2008;43(1):141–6.
Nadler EP, Reddy S, Isenalumhe A, et al. Laparoscopic adjustable gastric banding for morbidly obese adolescents affects android fat loss, resolution of comorbidities, and improved metabolic status. J Am Coll Surg. 2009;209(5):638–44.
Silva GM, Osório A, Pereira F, et al. Effect of laparoscopic adjustable gastric banding on modifiable cardiovascular risk factors in extremely obese adolescents. Obes Surg. 2012;22(6):991–4.
Dillard 3rd BE, Gorodner V, Galvani C, et al. Initial experience with the adjustable gastric band in morbidly obese US adolescents and recommendations for further investigation. J Pediatr Gastroenterol Nutr. 2007;45(2):240–6.
Angrisani L, Favretti F, Furbetta F, et al. Obese teenagers treated by Lap-Band System: the Italian experience. Surgery. 2005;138(5):877–81.
Silberhumer GR, Miller K, Kriwanek S, et al. Laparoscopic adjustable gastric banding in adolescents: the Austrian experience. Obes Surg. 2006;16(8):1062–7.
Khen-Dunlop N, Dabbas M, De Filippo G, et al. Primordial influence of post-operative compliance on weight loss after adolescent laparoscopic adjustable gastric banding. Obes Surg. 2016;26(1):98–104.
DuCoin C, Moon RC, Mulatre M, et al. Safety and effectiveness of Roux-en-Y gastric bypass in patients between the ages of 17 and 19. Obes Surg. 2015;25(3):464–9.
Olbers T, Gronowitz E, Werling M, et al. Two-year outcome of laparoscopic Roux-en-Y gastric bypass in adolescents with severe obesity: results from a Swedish Nationwide Study (AMOS). Int J Obes. 2012;36(11):1388–95.
Teeple E, Teich S, Schuster D, et al. Early metabolic improvement following bariatric surgery in morbidly obese adolescents. Pediatr Blood Cancer. 2012;58(1):112–6.
Tsamis D, Plastiras A, Natoudi M, et al. Impact of laparoscopic sleeve gastrectomy on weight loss and associated comorbidities in adolescents and young adults. J Laparoendosc Adv Surg Tech. 2015;25(12):971–5.
Alqahtani AR, Antonisamy B, Alamri H, et al. Laparoscopic sleeve gastrectomy in 108 obese children and adolescents aged 5 to 21 years. Ann Surg. 2012;256(2):266–73.
Alqahtani A, Elahmedi M, Qahtani AR. Laparoscopic sleeve gastrectomy in children younger than 14 years: refuting the concerns. Ann Surg. 2016;263(2):312–9.
Alqahtani AR, Elahmedi MO. Pediatric bariatric surgery: the clinical pathway. Obes Surg. 2015;25(5):910–21.
Widhalm K, Fritsch M, Widhalm H, et al. Bariatric surgery in morbidly obese adolescents: long-term follow-up. Pediatr Obes. 2011;6(S1):65–9.
Widhalm K, Dietrich S, Prager G, et al. Bariatric surgery in morbidly obese adolescents: a 4-year follow-up of ten patients. Pediatr Obes. 2008;3(S1):78–82.
Olbers T, Beamish AJ, Gronowitz E, et al. Laparoscopic Roux-en-Y gastric bypass in adolescents with severe obesity (AMOS): a prospective, 5-year, Swedish nationwide study. Lancet Diabetes Endocrinol. 2017;5(3):174–83.
Inge TH, Courcoulas AP, Jenkins TM, et al. Weight loss and health status 3 years after bariatric surgery in adolescents. N Engl J Med. 2016;374(2):113–23.
Reisler G, Tauber T, Afriat R, et al. Sibutramine as an adjuvant therapy in adolescents suffering from morbid obesity. Isr Med Assoc J. 2006;8(1):30.
Coles N, Birken C, Hamilton J. Emerging treatments for severe obesity in children and adolescents. BMJ. 2016;354:i4116.
Boland CL, Harris JB, Harris KB. Pharmacological management of obesity in pediatric patients. Ann Pharmacother. 2015;49(2):220–32.
Imaz I, Martínez-Cervell C, García-Álvarez EE, et al. Safety and effectiveness of the intragastric balloon for obesity. A meta-analysis. Obes Surg. 2008;18(7):841–6.
Schouten R, Rijs CS, Bouvy ND, et al. A multicenter, randomized efficacy study of the EndoBarrier Gastrointestinal Liner for presurgical weight loss prior to bariatric surgery. Ann Surg. 2010;251(2):236–43.
Black J, White B, Viner R, et al. Bariatric surgery for obese children and adolescents: a systematic review and meta-analysis. Obes Rev. 2013;14(8):634–44.
Malaki M. Sibutramine: a banned innocent antiobesity drug. J Pharm Negat Results. 2016;7(1):53.
James WPT, Caterson ID, Coutinho W, et al. Effect of sibutramine on cardiovascular outcomes in overweight and obese subjects. N Engl J Med. 2010;363(10):905–17.
Shapira B, Goldstein L, Reshef A, et al. A rare case of psychomotor disturbances linked to the use of an adulterated dietary supplement containing sibutramine. Clin Neuropharmacol. 2016;39(3):154–6.
Gibson LJ, Peto J, Warren JM, et al. Lack of evidence on diets for obesity for children: a systematic review. Int J Epidemiol. 2006;35(6):1544–52.
Partsalaki I, Karvela A, Spiliotis BE. Metabolic impact of a ketogenic diet compared to a hypocaloric diet in obese children and adolescents. J Pediatr Endocrinol Metab. 2012;25(7–8):697–704.
Reeves B, Deeks J, Higgins J, Wells G., on behalf of the Cochrane Non-Randomised Studies Methods Group. Chapter 13: including non-randomized studies. Cochrane handbook for systematic reviews of interventions.Version 2008;5(0).
Freedman DS, Sherry B. The validity of BMI as an indicator of body fatness and risk among children. Pediatrics. 2009;124(Suppl 1):S23–34.
McGovern L, Johnson JN, Paulo R, et al. Treatment of pediatric obesity: a systematic review and meta-analysis of randomized trials. J Clin Endocrinol Metab. 2008;93(12):4600–5.
McDonagh MS, Selph S, Ozpinar A, et al. Systematic review of the benefits and risks of metformin in treating obesity in children aged 18 years and younger. JAMA Pediatr. 2014;168(2):178–84.
O’Brien PE, Sawyer SM, Laurie C, et al. Laparoscopic adjustable gastric banding in severely obese adolescents: a randomized trial. JAMA. 2010;303(6):519–26.
Funding
Mr. Nicholas Penney is funded by the Diabetes Research and Wellness Foundation through the Sutherland-Earl Clinical Research Fellowship 2015.
Author information
Authors and Affiliations
Contributions
Study design: authors 2 and 5. Data search and collection: authors 1 and 3. Data analysis and interpretation: authors 1 and 2. Drafting of manuscript: author 1. Critical revision of manuscript: authors 2, 4 and 5.
Corresponding author
Ethics declarations
Competing Interests
The authors declare that they have no conflicts of interest.
Ethical Approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Informed Consent
Does not apply.
Additional information
The original article has been updated to corrected errors introduced during production.
Electronic supplementary material
ESM 1
(DOCX 1516 kb)
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Selvendran, S.S., Penney, N.C., Aggarwal, N. et al. Treatment of Obesity in Young People—a Systematic Review and Meta-analysis. OBES SURG 28, 2537–2549 (2018). https://doi.org/10.1007/s11695-018-3285-x
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11695-018-3285-x