Abstract
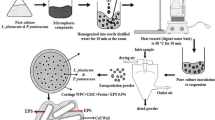
The present study aimed to identify the efficient cryoprotectants formulations on the survival of Lactobacillus plantarum CJLP133 microencapsulated in calcium alginate during freeze-drying. Trehalose, maltodextrin, sorbitol, sucrose, and soy peptone were selected to develop optimal formulations of cryoprotectants that were added to the calcium alginate microencapsulation using extrusion technology. The soy peptone after being combined with trehalose and maltodextrin was determined to have the highest cell viability than other combinations of sucrose and sorbitol during freeze-drying after microencapsulation (p ≤ 0.05). After storage for 12 weeks at 40 °C, the viability of microencapsulated L. plantarum was reduced by 1.03 log CFU mL− 1, while free cell viability was reduced by 1.68 log CFU mL− 1. Additionally, microencapsulated cells with cryoprotectants were resistant to simulated stomach–duodenum passage (SSDP) in vitro model, compared to free cells (p ≤ 0.05). This study highlighted that soy peptone was the most suitable cryoprotectant with trehalose and that the addition of maltodextrin during freeze-drying after microencapsulation of L. plantarum conferred enhanced survival during long-term storage at high temperatures and under adverse SSDP conditions.




Similar content being viewed by others
Change history
30 March 2023
A Correction to this paper has been published: https://doi.org/10.1007/s11694-023-01918-4
References
FAO WHO, Guidelines for the Evaluation of Probiotic in Food (FAO, Paris, 2001), pp. 1–11
P.G. Casey, G.D. Casey, G.E. Gardiner, M. Tangney, C. Stanton, R.P. Ross, G.F. Fitzgerald, Lett. Appl. Microbiol. 39, 431–438 (2004)
L.C. Su, C.W. Lin, M.J. Chen, Int. J. Agric. Technol. 60, 49–54 (2007)
H.K. Solanki, D.D. Pawar, D.A. Shah, V.D. Prajapati, G.K. Jani, A.M. Mulla, P.M. Thakar, Biomed Res. Int, 2013, 620719 (2013)
B. Sánchez, S. Delgado, A. Blanco-Míguez, A. Lourenço, M. Gueimonde, A. Margolles, Mol Nutr Food Res. 61 (2017)
X. Meng, C. Stanton, G. Fitzgerald, C. Daly, R. Ross, Food Chem. 106, 1406–1416 (2008)
T. Duong, R. Barrangou, W.M. Russell, T.R. Klaenhammer, Appl. Environ. Microbiol. 72, 1218–1225 (2006)
K. Li, B. Wang, W. Wang, G. Liu, W. Ge, M. Zhang, LWT 116, 108521 (2019)
A. Talwalkar, K. Kailasapathy, Int. J. Dairy. Sci. 86, 2537–2546 (2003)
Z. Hubálek, Cryobiology 46, 205–229 (2003)
A. Carvalho, J. Silva, P. Ho, P. Teixeira, F.X. Malcata, P. Gibbs, Biotechnol. Prog 20, 248–254 (2004)
R.I. Dave, N.P. Shah, Int. J. Dairy. Sci. 81, 2804–2816 (1998)
W. Krasaekoopt, B. Bhandari, H. Deeth, Int. Dairy. J. 13, 3–13 (2003)
R. Vaniski, S.C. da Silva, R.A. da Silva-Buzanello, C. Canan, D.A. Drunkler, J. Food Process. Preserv 45, 15364 (2021)
J.M. Mathara, U. Schillinger, C. Guigas, C. Franz, P.M. Kutima, S.K. Mbugua, Int. J. Food Microbiol. 126, 57–64 (2008)
P. Barajas-Álvarez, M. González-Ávila, H. Espinosa-Andrews, LWT 153, 112485 (2022)
C. Hill, F. Guarner, G. Reid, G.R. Gibson, D.J. Merenstein, B. Pot, L. Morelli, R.B. Canani, J.H. Flint, S. Salminen, C.P. Calder, E.M. Sanders, Nat. Rev. Gastroenterol. Hepatol. 11, 506–514 (2014)
J. Girón-Hernández, P. Gentile, M. Benlloch-Tinoco, Carbohydr. Polym. 271, 118429 (2021)
K.L. Liao, M.C. Yin, J. Agric. Food Chem. 48, 2266–2270 (2000)
N.P.R. Barro, L.M. da Silva, G. de Souza Hassemer, E. Franceschi, R.L. Cansian, A. Junges, G.T. Backes, J. Jamile, C. Rosicler, M. Mignoni, E. Valduga, Biointerface Res. Appl. Chem. 11, 11221–11232 (2020)
S. Nanasombat, N. Sriwong, KMITL Sci. Tech. J. 7 (2007)
A. Karina, P. Hanyung, K. Bobae, Y. Subin, J. Hyunjoo, K. Jin-Hak, J. Yosep, H.H. wilhelm, Korean J. Appl. Microbiol. Biotechnol. 49, 157–166 (2021)
S. Yeo, H.S. Shin, H.W. Lee, D. Hong, H. Park, W. Holzapfel, E.B. Kim, C.S. Huh, J. Microbiol. Biotechnol. 28, 718–731 (2018)
A. Chotiko, S. Sathivel, LWT - Food Sci. Technol. 59, 59–64 (2014)
K.B.P.K. Reddy, S.P. Awasthi, A.N. Madhu, S.G. Prapulla, Food Biotechnol. 23, 243–265 (2009)
G.F. de Valdéz, G.S. de Giori, A.A. de Ruiz Holgado, G. Oliver, Appl. Environ. Microbiol. 45, 302–304 (1983)
D. Rodrigues, S. Sousa, T. Rocha-Santos, J.P. Silva, J.M. Sousa Lobo, P. Costa, M.H. Amaral, M.M. Pintado, A.M. Gomes, F.X. Malcata, A.C.Feites. Int. Dairy J. 21, 869–876 (2011)
C. Desmond, C. Stanton, G.F. Fitzgerald, K. Collins, R. Paul Ross, Int. Dairy. J. 11, 801–808 (2001)
T. Tsvetkov, R. Brankova, Cryobiology 20, 318–323 (1983)
M.B. Akin, S. Akin, Food Chem. 100, 788–793 (2007)
A. Nag, S. Das, Int. J. Dairy. Technol. 66, 162–169 (2013)
R.M. Wang, N. Li, K. Zheng, J.F. Hao, FEMS Microbiol. Lett. (2018)
R.R. Mokarram, S.A. Mortazavi, M.B. Habibi Najafi, F. Shahidi, Int. Food Res. J. 42, 1040–1045 (2009)
Funding
This study was supported by the Korea Institute of Planning and Evaluation for Technology in Food, Agriculture, Forestry and Fisheries (IPET) through the High Value-added Food Technology Development Program, funded by the Ministry of Agriculture, Food and Rural Affairs (MAFRA) (121012-03).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Declarations
All authors declare that there are no conflicts of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Dong Joo Shin, Enkhtsatsral Elbegbayar contributed equally to this work.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Shin, D.J., Elbegbayar, E., Baek, Y. et al. Effects of different cryoprotectants on the viability of microencapsulated Lactobacillus plantarum CJLP133 during long-term storage. Food Measure 17, 3264–3271 (2023). https://doi.org/10.1007/s11694-023-01863-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11694-023-01863-2