Abstract
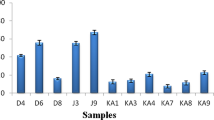
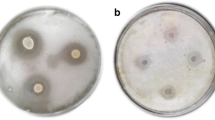
The concerns about synthetic preservatives on human health lead the food industry and scientists to seek natural agents from natural sustainable sources; including plants, animals, and microorganisms. In this study, it was aimed to characterise and enhance the bioactivity of microencapsulated cell free supernatant (CFS) of Lactobacillus plantarum FI 8595 by powdered propolis (PP) against fish spoilage bacteria and food-borne pathogens. CFS mainly consisted of n-hexadecanoic acid (27.44%) and 1-hexadecene (19.59%), while PP had primarily 2-furanmethanol (46.17%) and 2-ethyl hexanal (13.98%). Proteus mirabilis was the most resistant strain towards CFS, with inhibition zone of 8.43 mm. Addition of PP into CFS enhanced antimicrobial activity of lactobacilli CFS. Microencapsulated CFS with PP exerted higher inhibition zone (15 mm) than their pure form (9.33 mm) against Enterococcus faecalis. Staphylococcus aureus and Salmonella Paratyphi A exhibited stronger susceptibility versus pure and microencapsulated samples. Bacteriostatic effect of microencapsulated CFS was found at dose of 25 mg/ml for P. mirabilis, and 50 mg/ml for Pseudomonas luteola, E. faecalis, and P. damselae. All bacteria apart from P. luteola kept growing at 50 mg/ml dose of microencapsulated CFS, whereas microencapsulated CFS in combination with PP fully killed all bacteria at this dose. Results revealed that combinations of microencapsulated CFS and PP could be a good alternative to control microbial growth in food.

Similar content being viewed by others
References
T. Dinev, G. Beev, M. Tzanova, S. Denev, D. Dermendzhieva, A. Stoyanova, Bulg. J. Vet. Med. 21, 1–16 (2018)
F. Saeed, M. Afzaal, T. Tufail, A. Ahmad, Act. Antimicrob. Food Packag. (2019)
X. Xu, A. Liu, S. Hu, I. Ares, M.R. Martínez-Larrañaga, X. Wang, A. Anadón, M.A. Martínez, Food. Chem. 353, 129488 (2021)
J. Mahmud, R.A. Khan, Adv. Microbiol. 8, 894–916 (2018)
O. Ogunkalu, R. Khalily, Y. Salci, Eurasian J. Agric. Res. 2, 74–84 (2008)
C. Miranda, D. Contente, G. Igrejas, S.P.D.A. Câmara, M.D.L.E. Dapkevicius, P. Poeta, Foods. 10, 1–21 (2021)
T.H. Lin, T.M. Pan, J. Microbiol. Immunol. Infect. 52, 409–417 (2019)
E. Mani-López, D. Arrioja-Bretón, A. López-Malo, Compr. Rev. Food Sci. Food Saf. 21, 604–641 (2022)
A. Ołdak, D. Zielińska, A. Rzepkowska, D. Kołożyn-Krajewska, BioMed. Res. Int. 2017, 1–10 (2017)
Z. Muhammad, R. Ramzan, A. Abdelazez, A. Amjad, M. Afzaal, S. Zhang, S. Pan, Pathogens 8, 1–20 (2019)
A. Cebeci, C. Gürakan, Food Microbiol. 20, 511–518 (2003)
D.S. Dezmirean, C. Paşca, A.R. Moise, O. Bobiş, Plants 10, 1–20 (2021)
M.S. Almuhayawi, Saudi J. Biol. Sci. 27, 3079–3086 (2020)
D. MilojkovićOpsenica, P. Ristivojević, J. Trifković, I. Vovk, D. Lušić, Ž Tešić, J. Chromatogr. Sci. 54, 1077–1083 (2016)
K. Pobiega, K. Kraśniewska, D. Derewiaka, M. Gniewosz, J. Food Sci. Technol 56, 5386–5395 (2019)
K. Huang, Y. Yuan, X. Baojun, Food Rev. Int. (2021). https://doi.org/10.1080/87559129.2021.1963978
N. Sharif, S. Khoshnoudi-Nia, S.M. Jafari, Food Res. Int. 132, 1–18 (2020)
J. Castro-Rosas, C.R. Ferreira-Grosso, C.A. Gómez-Aldapa, E. Rangel-Vargas, M.L. Rodríguez-Marín, F.A. Guzmán-Ortiz, R.N. Falfan-Cortes, Food Res. Int. 102, 575–587 (2017)
L. Pachuau, P.K. Roy, J.H. Zothantluanga, S. Ray, S. Das, in Bioactive Natural Products for Pharmaceutical Applications (Springer, Cham, 2021), pp. 687–714
G.C. Raddatz, C.R.D. Menezes, Ciênc Rural 51, 1–8 (2021)
A. Burgut, J. Food Saf. 41, e12863 (2021)
E. Kuley, H. Yazgan, Y. Özogul, Y. Ucar, M. Durmus, G. Özyurt, D. Ayas, Food Biosci. 44, 101417 (2021)
E. Kuley, M. Durmus, Y. Ucar, A.R. Kosker, E.T.A. Tumerkan, J.M. Regenstein, F. Ozogul, Food Biosci. 24, 127–136 (2018)
M.Y. Lin, C.L. Yen, J. Agric. Food Chem. 47, 1460–1466 (1999)
F. Marcela, C. Lucía, F. Esther, M. Elena, J. Encapsulation Adsorpt. Sci. 6, 1–8 (2016)
N. Hwanhlem, T. Ivanova, T. Haertl´e, E. Jaffr`es, X. Dousset, LWT-Food Sci. Technol. 82, 170–175 (2017)
S.M.B. Hashemi, D. Jafarpour, J. Food Process. Preserv. 44, e14651 (2020)
Clinical and Laboratory Standards Institute, Methods for dilution antimicrobial susceptibility tests for bacteria that grow aerobically, Wayne, PA: CLSI, (2008)
Y. Li, B. Tang, J. Chen, P. Lai, Food Sci. Technol. 38, 530–536 (2017)
K. MisSolval, J.D. Bankston, P.J. Bechtel, S. Sathivel, J. Food Sci. 81(3), 600–609 (2016)
A.F. Yeşilsu, G. Özyurt, J. Food Eng. 240, 171–182 (2019)
R.V. Fernandes, S.V. Borges, D.A. Botrel, Carbohydr. Polym. 101, 524–532 (2014)
M. Moradi, S.A. Kousheh, H. Almasi, A. Alizadeh, J.T. Guimarães, N. Yılmaz, A. Lotfi, Compr. Rev. Food Sci. Food Saf. 19, 3390–3415 (2020)
F. Salleh, M.N. Lani, N.A. Kamaruding, T.Z.T. Chilek, N. Ismail, Appl. Food Biotechnol. 8, 121–132 (2021)
U. George-Okafor, U. Ozoani, F. Tasie, K. Mba-Omeje, Sci. Afr. 8, e00395 (2020)
M.C. Marcucci, Apidologie 26, 83–99 (1995)
D. Devequi-Nunes, B.A.S. Machado, G.D.A. Barreto, J. Rebouças Silva, D.F. da Silva, J.L.C. Rochada, H.N. Brandao, V.M. Borges, M.A. Umsza-Guez, PLoS ONE 13(12), 1–20 (2018)
S. Silici, S. Kutluca, J. Ethnopharmacol. 99, 69–73 (2005)
W. Liu, L. Miao, X. Li, Z. Xu, Coord. Chem. Rev. 429, 213646 (2021)
I. Przybyłek, T.M. Karpiński, Molecules 24, 1–17 (2019)
E.E. Imade, S.E. Omonigho, O.O. Babalola, B.J. Enagbonma, Ann. Microbiol. 71, 1–14 (2021)
H. Yazgan, Eur. J. Sci. Technol. 20, 485–489 (2020)
I.A.A. Raheem, A.A. Razek, A.A. Elgendy, N.M. Saleh, M.I. Shaaban, F.K. Abd El-Hady, Int. J. Nanomed. 14, 8379–8398 (2019)
E.M. Muli, J.M. Maingi, J. Venom. Anim. Toxins Incl. Trop. Dis. 13, 655–663 (2007)
M.G. Shehata, F.T. Ahmad, A.N. Badr, S.H. Masry, S. El-Sohaimy, Ann. Agric. Sci. 65, 209–217 (2020)
A.G. Evangelista, J.A.F. Corrêa, J.V.G. Dos Santos, E.H.C. Matté, M.M. Milek, G.C. Biauki, L.B. Costa, F.B. Luciano, Microbiology 167, 001102 (2021)
G. Casillas-Vargas, C. Ocasio-Malavé, S. Medina, C. Morales-Guzmán, R.G. Del Valle, N.M. Carballeira, D.J. Sanabria-Ríos, Prog. Lipid Res. 82, 1–10 (2021)
A.H.C. Lam, N. Sandoval, R. Wadhwa, J. Gilkes, T.Q. Do, W. Ernst, S.M. Chiang, H.H. Kosina Xu, G. Fujii, E. Porter BMC Res. Notes 9, 1–11
S. Yogeswari, S. Ramalakshmi, R. Neelavathy, J.Y. Muthumary, Glob. J. Pharmacol. 6, 65–71 (2012)
J.A. Hinton, K.D. Ingram, J. Food Protect. 63, 1282–1286 (2000)
F.R. Kalt, I.E. Cock, Pharmacogn Mag. 10, 37–49 (2014)
F. Patrignani, L. Iucci, N. Belletti, F. Gardini, M.E. Guerzoni, R. Lanciotti, Int. J. Food Microbiol. 123, 1–8 (2008)
K.M. Park, H.J. Kim, J.Y. Choi, M. Koo, Foods 10, 1–13 (2021)
N. Ziklo, M. Bibi, P. Salama, Cosmetics 8, 1–18 (2021)
Acknowledgements
The author would like to thank the academic staff of department of Seafood and Processing Technology, Faculty of Fisheries, Cukurova University (Adana, Turkey), who provided support in carrying out the work.
Author information
Authors and Affiliations
Contributions
AB: Investigation, conceptualization, methodology, writing–original draft, review & editing.
Corresponding author
Ethics declarations
Conflict of interest
The author wishes to confirm that there are no known conflicts of interest associated with this publication and there has been no significant financial support for this work that could have influenced its outcome.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Burgut, A. Characterization and microencapsulation of Lactobacillus plantarum FI 8595 cell free metabolites with enhanced antimicrobial property by powdered propolis. Food Measure 16, 4355–4363 (2022). https://doi.org/10.1007/s11694-022-01524-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11694-022-01524-w