Abstract
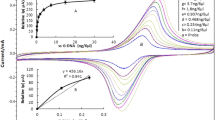
To effectively cut off the transmission of Brucella to humans through milk, a novel biosensor for detection of Brucella was constructed using cysteamine functionalized nanogold (Cys-Au) and 4-Mercaptobenzoic acid (4-MBA) modified gold electrode as the platform. The morphology of the prepared nanomaterials was characterized by laser particle sizer, XPS and SEM, and the results showed the 4-MBA film was modified on the gold electrode surface, and the diameter of successfully prepared nanogold and Cys-Au are about 55 and 65 nm, respectively. The electrochemical performance of the biosensor was tested in Brucella antigen solution by square wave voltammetry (SWV). The results showed the reproducibility and stability of the biosensor were good, the spiked recovery in milk was in the range of 95.12–105.27%, and the minimum detection limit (S/N = 3) was 5.12 × 102 cfu/mL with a linear range from 1.6 × 102 to 1.6 × 108 cfu/mL. In addition, the minimum detection limit was reduced from 5.12 × 102 cfu/mL to 7.0 × 101 cfu/mL after processing the SWV data by second-order derivative transformation, which showed Second-order derivative transformation is very helpful to further optimize the performance of the sensor. Compared with the previously reported test methods, the prepared biosensors have a wider linear range and lower limit of detection (LOD). Therefore, the constructed biosensor can be used as a promising tool for the trace detection of Brucella in milk samples.







Similar content being viewed by others
References
H.G. Garcell, E.G. Garcia, P.V. Pueyo, I.R. Martín, A.V. Arias, R.N.A. Serrano, Outbreaks of brucellosis related to the consumption of unpasteurized camel milk. J. Infect. Public Health 9, 523–527 (2016)
M.Z. Khan, M. Zahoor, An overview of brucellosis in cattle and humans, and its serological and molecular diagnosis in control strategies. Trop. Med. Infect. disease 3, 65 (2018)
V. Gupta, N. Shivasharanappa, V. Kumar, A. Kumar, Diagnostic evaluation of serological assays and different gene based PCR for detection of Brucella melitensis in goat. Small Ruminant Research 117, 94–102 (2014)
S. Sabour, M. Arzanlou, F. Jeddi, T. Azimi, S. Hosseini-Asl, A. Naghizadeh-Baghi, H.P. Dogaheh, Evaluating the efficiency of TaqMan real-time PCR and serological methods in the detection of Brucella spp. in clinical specimens collected from suspected patients in Ardabil, Iran. J. Microbiol. Methods 175, 105982 (2020)
M.M. Gwida, A.H. El-Gohary, F. Melzer, H. Tomaso, U. Rösler, U. Wernery, R. Wernery, M.C. Elschner, I. Khan, M. Eickhoff, Comparison of diagnostic tests for the detection of Brucella spp. in camel sera. BMC Res. Notes 4, 1–7 (2011)
P. Oleksandra, S. Tyler, B. Tuhina, S. Santimukul, A Comparison of Optical, Electrochemical, Magnetic, and Colorimetric Point-of-Care Biosensors for Infectious Disease Diagnosis. Acs Infectious Diseases 2018:acsinfecdis.8b00023-
A. Gattani, S.V. Singh, A. Agrawal, M.H. Khan, P. Singh, Recent progress in electrochemical biosensors as point of care diagnostics in livestock health. Anal. Biochem. 579, 25–34 (2019)
H. Sohrabi, M.R. Majidi, O. Arbabzadeh, P. Khaaki, S. Pourmohammad, A. Khataee, Y. Orooji, Recent advances in the highly sensitive determination of zearalenone residues in water and environmental resources with electrochemical biosensors. Environ. Res. 204, 112082 (2022)
H. Karimi-Maleh, Y. Orooji, F. Karimi, M. Alizadeh, M. Baghayeri, J. Rouhi, S. Tajik, H. Beitollahi, S. Agarwal, V.K. Gupta, A critical review on the use of potentiometric based biosensors for biomarkers detection. Biosens. Bioelectron. 184, 113252 (2021)
M. Mehmandoust, N. Erk, C. Karaman, O. Karaman, An electrochemical molecularly imprinted sensor based on CuBi2O4/rGO@ MoS2 nanocomposite and its utilization for highly selective and sensitive for linagliptin assay. Chemosphere 291, 132807 (2022)
H. Wu, Y. Zuo, C. Cui, W. Yang, H. Ma, X. Wang, Rapid quantitative detection of brucella melitensis by a label-free impedance immunosensor based on a gold nanoparticle-modified screen-printed carbon electrode. Sensors 13, 8551–8563 (2013)
L. Chen, S. Zhao, M. Liu, P. Wu, C. Chen, A novel label-free electrochemical immunosensor modified by glutathione and hyaluronic acid for the ultrasensitive and ultraselective detection of brucellosis in dilute serum. Sens. Actuators B 287, 510–516 (2019)
A.K. Gupta, V.K. Rao, D.T. Selvam, A. Kumar, R. Jain, Amperometric immunosensor of Brucella abortus CE-protein antigen shows post-zone phenomena. J. Electroanal. Chem. 717, 83–89 (2014)
J. Mohanraj, D. Durgalakshmi, R.A. Rakkesh, S. Balakumar, S. Rajendran, H. Karimi-Maleh, Facile synthesis of paper based graphene electrodes for point of care devices: A double stranded DNA (dsDNA) biosensor. J. Colloid Interface Sci. 566, 463–472 (2020)
K. Shiping, Q. Jianying, NO2- Sensor Based on Poly (Diallyldimethylammonium Chloride) and Mercaptoaceti Acid Modified Gold Electrode Through Electrostatic Self—Assembly. Anal. test techniques instruments 24, 91–96 (2018)
Q. Jing, L. Kaoqi, K. Weinjun, N. Lingmei. Research of biosensor and its application based on the mercaptoacetic acid self-assembled monolayer. CHEMICAL SENSORS 2013:45 – 8
H. Wang, H. Ohnuki, H. Endo, M. Izumi, Preparation of amperometric glucose biosensor based on 4-mercaptobenzoic acid. Phys. Procedia 14, 2–6 (2011)
S. Zexuan, L. Xiao, K. Weinjun, N. Lingmei, The electrochemical behavior of 5-hydroxytryptamine at mercaptobenzoic acid modified electrode and its application. CHEMICAL SENSORS 2018;2
S.M. Rosendahl, I.J. Burgess, Electrochemical and infrared spectroscopy studies of 4-mercaptobenzoic acid SAMs on gold surfaces. Electrochim. Acta 53, 6759–6767 (2008)
H. Karimi-Maleh, A. Khataee, F. Karimi, M. Baghayeri, L. Fu, J. Rouhi, C. Karaman, O. Karaman, R. Boukherroub, A green and sensitive guanine-based DNA biosensor for idarubicin anticancer monitoring in biological samples: A simple and fast strategy for control of health quality in chemotherapy procedure confirmed by docking investigation. Chemosphere 291, 132928 (2022)
M. Mehmandoust, N. Erk, O. Karaman, F. Karimi, M. Bijad, C. Karaman, Three-dimensional porous reduced graphene oxide decorated with carbon quantum dots and platinum nanoparticles for highly selective determination of azo dye compound tartrazine. Food Chem. Toxicol. 158, 112698 (2021)
M. Mehmandoust, N. Erk, M. Alizadeh, S. Salmanpour, Voltammetric carbon nanotubes based sensor for determination of tryptophan in the milk sample. J. Food Meas. Charact. 15, 5288–5295 (2021)
H. Karimi-Maleh, F. Karimi, L. Fu, A.L. Sanati, M. Alizadeh, C. Karaman, Y. Orooji, Cyanazine herbicide monitoring as a hazardous substance by a DNA nanostructure biosensor. J. Hazard. Mater. 423, 127058 (2022)
H. Karimi-Maleh, M.L. Yola, N. Atar, Y. Orooji, F. Karimi, P.S. Kumar, J. Rouhi, M. Baghayeri, A novel detection method for organophosphorus insecticide fenamiphos: Molecularly imprinted electrochemical sensor based on core-shell Co3O4@ MOF-74 nanocomposite. J. Colloid Interface Sci. 592, 174–185 (2021)
Y. Yang, M. Jiang, K. Cao, M. Wu, C. Zhao, H. Li, C. Hong, An electrochemical immunosensor for CEA detection based on Au-Ag/rGO@ PDA nanocomposites as integrated double signal amplification strategy. Microchem. J. 151, 104223 (2019)
M. Yanyan, H. Yayu, W. LIyan, Chemical preparation method of nanogold and its application in biomedical field. Shandong Industrial Technology 2014:1
P. Chauhan, A.N. Raja, R. Jain, Nanogold modified glassy carbon sensor for the quantification of phytoestrogenchlorogenic acid. Surf. Interfaces 19, 100536 (2020)
H. Guan, J. Yu, D. Chi, Label-free colorimetric sensing of melamine based on chitosan-stabilized gold nanoparticles probes. Food Control 32, 35–41 (2013)
Z.-E. Shi, S.-H. Liu, C.-H. Tsai, C.-W. Li, C.-P. Chen, Y.-H. Yu, Enhancing charge transport performance of perovskite solar cells by using reduced graphene oxide-cysteine/nanogold hybrid material in the active layer. FlatChem 28, 100254 (2021)
H. Majdi, R. Salehi, M. Pourhassan-Moghaddam, S. Mahmoodi, Z. Poursalehi, S. Vasilescu, Antibody conjugated green synthesized chitosan-gold nanoparticles for optical biosensing. Colloid and Interface Science Communications 33, 100207 (2019)
L. Lin, Z. Xiaoqiang, Z. Yu, P. Yuebu. Conjugation of antibody on carboxyl-SPIO nanoparticles based on EDC/ sulfo-NHS. ACTA UNIVERSITATIS MEDICINALIS NANJING(Natural Science) 2013;033:480-4
T.-C. Tsai, C.-W. Liu, Y.-C. Wu, N.A.P. Ondevilla, M. Osawa, H.-C. Chang, In situ study of EDC/NHS immobilization on gold surface based on attenuated total reflection surface-enhanced infrared absorption spectroscopy (ATR-SEIRAS). Colloids Surf., B 175, 300–305 (2019)
N.S. Ferreira, M.G.F. Sales, Disposable immunosensor using a simple method for oriented antibody immobilization for label-free real-time detection of an oxidative stress biomarker implicated in cancer diseases. Biosens. Bioelectron. 53, 193–199 (2014)
A.-J. Wang, X.-Y. Zhu, Y. Chen, P.-X. Yuan, X. Luo, J.-J. Feng, A label-free electrochemical immunosensor based on rhombic dodecahedral Cu3Pt nanoframes with advanced oxygen reduction performance for highly sensitive alpha-fetoprotein detection. Sens. Actuators B 288, 721–727 (2019)
A.M.P.C. B CASA, R.A.M. C, C FLM, B MDLOA. Self-assembled monolayers of mercaptobenzoic acid and magnetite nanoparticles as an efficient support for development of tuberculosis genosensor. J. Colloid Interface Sci. 433, 141–148 (2014)
Xu Junjun, W. Tiantian, X. Huiting, Y. CUishu, D. Danwen, Construction of a Novel Hydrogen Peroxide Sensor and Its Application in Milk. Sci. Technol. Food Ind. 41, 261–267 (2020)
S. Kasturi, Y. Eom, S.R. Torati, C. Kim, Highly sensitive electrochemical biosensor based on naturally reduced rGO/Au nanocomposite for the detection of miRNA-122 biomarker. J. Ind. Eng. Chem. 93, 186–195 (2021)
Y. Yuan, Y. Wang, H. Wang, S. Hou, Gold nanoparticles decorated on single layer graphene applied for electrochemical ultrasensitive glucose biosensor. J. Electroanal. Chem. 855, 113495 (2019)
L. Zhao, Y. Wang, Z. Li, Y. Deng, X. Zhao, Y. Xia, Facile synthesis of chitosan-gold nanocomposite and its application for exclusively sensitive detection of Ag + ions. Carbohydr. Polym. 226, 115290 (2019)
Y. Zhou, S. Wang, K. Zhang, X. Jiang, Visual detection of copper (II) by azide-and alkyne‐functionalized gold nanoparticles using click chemistry. Angew. Chem. Int. Ed. 47, 7454–7456 (2008)
D.M. Luna, K.Y. Avelino, M.T. Cordeiro, C.A. Andrade, M.D. Oliveira, Electrochemical immunosensor for dengue virus serotypes based on 4-mercaptobenzoic acid modified gold nanoparticles on self-assembled cysteine monolayers. Sens. Actuators B 220, 565–572 (2015)
C. Tao, Research on the Charged Characteristics of Nanoparticles and Properties of Polyethylene Nanocomposites (Harbin University of Science and Technology, 2014)
A.J. Bard, RF-L. Principles and applications of electrochemical methods (Chemical Industry Publisher, 2005)
S. Khan, Z. Ansari, H. Seo, S. Ansari, Synthesis and application of Cu-doped nickel and zirconium oxide nanoparticles as Brucella abortus electrochemical device development. Sens. Lett. 16, 267–276 (2018)
S. Khan, Z. Ansari, O.Y. Alothman, H. Fouad, S. Ansari, Application of amine and copper doped magnesium oxide nanoparticles in electrochemical immunosensors for detecting brucella abortus. Nanosci. Nanatechnol. Lett. 9, 1656–1664 (2017)
L. BenXiang, W. Ling, D. XinRong, Application of second derivative pretreatment in quantitative analysis using mid-infrared spectrum. Chin. J. Anal. Lab. 27, 9–12 (2008)
Z. Dequan, Determination of Benzoyl Peroxide in Flour by Quadratic Derivative Ultraviolet Spectrophotometry. Chinese Journal of Control of Endemic Diseases 2014:462-
L. Yunai, Z. Tianmin, Establishment of second-order derivative differential pulse polarimetry and its application in drug analysis. Acta Pharm. sinica 27, 157–160 (1992)
C. Jiemei, P. Tao, C. Xingdan, Application of second derivative spectrum prepares in quantification measuring glucose-6-phosphate and fructose-6-phosphate using a FTIR /ATR method. Opt. Precision Eng. 14, 1–7 (2006)
A. Cobelo-García, J. Santos-Echeandía, D.E. López-Sánchez, C. Almécija, D. Omanović, Improving the voltammetric quantification of ill-defined peaks using second derivative signal transformation: example of the determination of platinum in water and sediments. Anal. Chem. 86, 2308–2313 (2014)
Z. Guangzhu, Y. Fengjie, W. Cuizhen, Lifting transform via Savitsky-Golay filter predictor and application of denoising. Journal of Coal ence & Engineering 2006:66–9
L. Song, J. Li, S. Hou, X. Li, S. Chen, Establishment of loop-mediated isothermal amplification (LAMP) for rapid detection of Brucella spp. and application to milk and blood samples. J. Microbiol. Methods 90, 292–297 (2012)
G. Bayramoglu, V.C. Ozalp, M. Oztekin, M.Y. Arica, Rapid and label-free detection of Brucella melitensis in milk and milk products using an aptasensor. Talanta 200, 263–271 (2019)
L. Che, C. Qi, W. Bao, X. Ji, J. Liu, N. Du, L. Gao, K. Zhang, Li Y-x. Monitoring the course of Brucella infection with qPCR-based detection. Int. J. Infect. Dis. 89, 66–71 (2019)
R. Hans, P.K. Yadav, P.K. Sharma, M. Boopathi, D. Thavaselvam, Development and validation of immunoassay for whole cell detection of Brucella abortus and Brucella melitensis. Sci. Rep. 10, 1–13 (2020)
A.D. Dursun, B.A. Borsa, G. Bayramoglu, M.Y. Arica, V.C. Ozalp, Surface plasmon resonance aptasensor for Brucella detection in milk. Talanta 239, 123074 (2022)
Acknowledgements
This work was supported from the project of Youth Fund for Science and Technology Research in Hebei Universities (grant number: QN2020194), the Project of Youth Fund for National Natural Science Foundation of China (grant number:32001791) and the National Natural Science Foundation of China (grant number 30871445).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Chen, H., Liu, H., Cui, C. et al. Highly sensitive detection of Brucella in milk by cysteamine functionalized nanogold/4-Mercaptobenzoic acid electrochemical biosensor. Food Measure 16, 3501–3511 (2022). https://doi.org/10.1007/s11694-022-01428-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11694-022-01428-9