Abstract
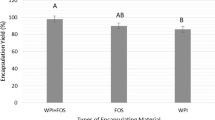
The objective of the present study was to evaluate the effect of microbial exopolysaccharide (EPS) encapsulation of lactic acid bacteria on enhancing their viability during exposure to the simulated conditions of the gasterointestinal tract. Lactobacillus plantarum (NR_104573.1) and Pediococcus pentosaceus (NR_042058.1) isolated from wheat bran sourdough were encapsulated by spray-drying with various ratios of EPS, whey protein concentrate, carboxymethyl cellulose and pectin. The viability, kinetics and survival under stress conditions were compared between the samples after 120 min of incubation and over 28 days of storage. HPLC was used for compositional assessment in terms of monosaccharide constituents, revealing that glucose, arabinose and xylose were the major components of the EPS produced by L. plantarum and P. pentosaceus. ANOVA demonstrated a significantly reduced logarithmic cycle of bacterial population in the control samples and free cells compared to the encapsulated L. plantarum and P. pentosaceus after 2 h in simulated gastric fluid conditions and bile salt solution. Encapsulation yields in the presence of WPC, CMC and pectin with and without EPS were about 85 and 80% for L. plantarum, and it was 81 and 75% for P. pentosaceus, respectively. Also, the viability number of L. plantarum and P. pentosaceus free cells decreased over 28 days of storage from 12.41 to 7.28 and 12.11 to 6.96 log Cfu/mL, respectively. Finally, by assessing the kinetics of the bacteria with three mathematical models, the Ritger–Peppas kinetics model was found to be a suitable correlation model for the data.





Similar content being viewed by others
References
I.W. Sutherland, Novel and established applications of microbial polysaccharides. Trends Biotechnol. 16, 41–46 (1998)
B. Vu, M. Chen, R.J. Crawford, E.P. Ivanova, Bacterial extracellular polysaccharides involved in biofilm formation. Molecules. 14, 2535–2554 (2009)
C. Liu, J. Lu, L. Lu, Y. Liu, F. Wang, M. Xiao, Isolation, structural characterization and immunological activity of an exopolysaccharide produced by Bacillus licheniformis 8-37-0-1. Bioresour. Technol. 101, 5528–5533 (2010)
A. Abedfar, M. Hossininezhad, A. Sadeghi, M. Raeisi, J. Feizy, Investigation on “spontaneous fermentation” and the productivity of microbial exopolysaccharides by Lactobacillus plantarum and Pediococcus pentosaceus isolated from wheat bran sourdough. LWT Food Sci. Technol. 96, 686–693 (2018)
M.Y. Chen, W. Zheng, Q.Y. Dong, Z.H. Li, L.E. Shi, Z.X. Ananta, Activity of encapsulated Lactobacillus bulgaricus in alginate-whey protein microspheres. Braz. Arch. Biol. Technol. 57, 736–741 (2014)
L.E. Shi, Z.H. Li, D.T. Li, M. Xu, H.Y. Chen, Z.L. Zhang, Z.X. Tang, Encapsulation of probiotic Lactobacillus bulgaricus in alginate–milk microspheres and evaluation of the survival in simulated gastrointestinal conditions. J. Food Eng. 117, 99–104 (2013)
A.K. Anal, W.F. Stevens, C. Remunan-Lopez, Ionotropic cross-linked chitosan microspheres for controlled release of ampicillin. Int. J. Pharm. 312, 166–173 (2006)
E. Ananta, M. Volkert, D. Knorr, Cellular injuries and storage stability of spray-dried Lactobacillus rhamnosus GG. Int. Dairy J. 15, 399–409 (2005)
L.E. Shi, W. Zheng, Y. Zhang, Z.X. Tang, Milk-alginate microspheres: Protection and delivery of Enterococcus faecalis HZNU P2. LWT Food Sci. Technol. 65, 840–844 (2016)
Q.Y. Dong, M.Y. Chen, Y. Xin, X.Y. Qin, Z. Cheng, L.E. Shi, Z.X. Tang, Alginate-based and protein‐based materials for probiotics encapsulation: a review. Int. J. Food Sci. Technol. 48, 1339–1351 (2013)
S. Rokka, P. Rantamaki, Protecting probiotic bacteria by microencapsulation: challenges for industrial applications. Eur. Food Res. Technol. 231, 1–12 (2010)
B.M. Corcoran, R.P. Ross, G.F. Fitzgerald, C. Stanton, Comparative survival of probiotic lactobacilli spray-dried in the presence of prebiotic substances. J. Appl. Microbiol. 96, 1024–1039 (2004)
M. Girard, S.L. Turgeon, S.F. Gauthier, Interbiopolymer complexing between β-lactoglobulin and low-and high-methylated pectin measured by potentiometric titration and ultrafiltration. Food Hydrocoll. 16, 585–591 (2002)
F. Dal Bello, C.I. Clarke, L.A.M. Ryan, H. Ulmer, T.J. Schober, K. Ström, J. Sjögren, D. Van Sinderen, J. Schnürer, E.K. Arendt, Improvement of the quality and shelf life of wheat bread by fermentation with the antifungal strain Lactobacillus plantarum FST 1.7. J Cereal Sci. 45, 309–318 (2007)
Y. Boza, L.P. Neto, F.A.A. Costa, A.R.P. Scamparini, Exopolysaccharide production by encapsulated Beijerinckia cultures. Process Biochem. 39, 1201–1209 (2004)
R. Tallon, P. Bressollier, M.C. Urdaci, Isolation and characterization of two exopolysaccharides produced by Lactobacillus plantarum EP56. Res. Microbiol. 154, 705–712 (2003)
Y. Lv, X. Yang, Y. Zhao, Y. Ruan, Y. Yang, Z. Wang, Separation and quantification of component monosaccharides of the tea polysaccharides from Gynostemma pentaphyllum by HPLC with indirect UV detection. Food Chem 112, 742–746 (2009)
H. Stepan, E. Staudacher, Optimization of monosaccharide determination using anthranilic acid and 1-phenyl-3-methyl-5-pyrazolone for gastropod analysis. Anal. Biochem. 418, 24–29 (2011)
M.E. Rodríguez-Huezo, R. Durán-Lugo, L.A. Prado-Barragán, F. Cruz-Sosa, C. Lobato-Calleros, J. Alvarez-Ramírez, E.J. Vernon-Carter, Pre-selection of protective colloids for enhanced viability of Bifidobacterium bifidum following spray-drying and storage, and evaluation of aguamiel as thermoprotective prebiotic. Food Res. Int. 40, 1299–1306 (2007)
R. Altamirano-Fortoul, R. Moreno-Terrazas, A. Quezada-Gallo, C.M. Rosell, Viability of some probiotic coatings in bread and its effect on the crust mechanical properties. Food Hydrocoll. 29, 166–174 (2012)
Z. Tang, X. Huang, P.M. Sabour, J.R. Chambers, Q. Wang, Preparation and characterization of dry powder bacteriophage K for intestinal delivery through oral administration. LWT Food Sci. Technol. 60, 263–270 (2015)
L.E. Shi, Z.H. Li, Z.L. Zhang, T.T. Zhang, W.M. Yu, M.L. Zhou, Z.X. Tang, Encapsulation of Lactobacillus bulgaricus in carrageenan-locust bean gum coated milk microspheres with double layer structure. LWT Food Sci. Technol. 54, 147–151 (2013)
A. Picot, C. Lacroix, Encapsulation of bifidobacteria in whey protein-based microcapsules and survival in simulated gastrointestinal conditions and in yoghurt. Int. Dairy J. 14, 505–515 (2004)
H. Michida, S. Tamalampudi, S.S. Pandiella, C. Webb, H. Fukuda, A. Kondo, Effect of cereal extracts and cereal fiber on viability of Lactobacillus plantarum under gastrointestinal tract conditions. Biochem. Eng. J. 28, 73–78 (2006)
Y. Ma, J.C. Pacan, Q. Wang, P.M. Sabour, X. Huang, Y. Xu, Enhanced alginate microspheres as means of oral delivery of bacteriophage for reducing Staphylococcus aureus intestinal carriage. Food Hydrocoll. 26, 434–440 (2012)
W.P. Charteris, P.M. Kelly, L. Morelli, J.K. Collins, Development and application of an in vitro methodology to determine the transit tolerance of potentially probiotic Lactobacillus and Bifidobacterium species in the upper human gastrointestinal tract. J. Appl. Microbiol. 84, 759–768 (1998)
Y. He, Z. Wu, L. Tu, Y. Han, G. Zhang, C. Li, Encapsulation and characterization of slow-release microbial fertilizer from the composites of bentonite and alginate. Appl Clay Sci. 109, 68–75 (2015)
L.X. Pan, X.J. Fang, Z. Yu, Y. Xin, X.Y. Liu, L.E. Shi, Z.X. Tang, Encapsulation in alginate–skim milk microspheres improves viability of Lactobacillus bulgaricus in stimulated gastrointestinal conditions. Int. J. Food Sci. Nutr. 64, 380–384 (2013)
S. Benita, Kinetic model identification of drug release from microcapsules using the nonlinear regression search procedure. Appl. Biochem. Biotechnol. 10, 255–258 (1984)
T. Higuchi, Mechanism of sustained-action medication. Theoretical analysis of rate of release of solid drugs dispersed in solid matrices. J. Pharm. Sci. 52, 1145–1149 (1963)
P.L. Ritger, N.A. Peppas, A simple equation for description of solute release I. Fickian and non-Fickian release from non-swellable devices in the form of slabs, spheres, cylinders or discs. J. Control. Release. 5, 23–36 (1987)
S. Argin, P. Kofinas, Y.M. Lo, The cell release kinetics and the swelling behavior of physically crosslinked xanthan–chitosan hydrogels in simulated gastrointestinal conditions. Food Hydrocoll. 40, 138–144 (2014)
H. İspirli, E. Dertli, Isolation and identification of exopolysaccharide producer lactic acid bacteria from Turkish yogurt. J Food Process. Preserv. 42, 13351 (2018)
L. Ai, Q. Guo, H. Ding, B. Guo, W. Chen, S.W. Cui, Structure characterization of exopolysaccharides from Lactobacillus casei LC2W from skim milk. Food Hydrocoll. 56, 134–143 (2016)
Y. Wang, C. Li, P. Liu, Z. Ahmed, P. Xiao, X. Bai, Physical characterization of exopolysaccharide produced by Lactobacillus plantarum KF5 isolated from Tibet Kefir. Carbohydr. Polym. 82, 895–903 (2010)
G. Hebrard, V. Hoffart, E. Beyssac, J.M. Cardot, M. Alric, M. Subirade, Coated whey protein/alginate microparticles as oral controlled delivery systems for probiotic yeast. J. Microencapsul. 27, 292–302 (2010)
M. Hosseini Nezhad, M.A. Hussain, M.L. Britz, Stress responses in probiotic Lactobacillus casei. Crit. Rev. Food Sci. Nutr. 55, 740–749 (2015)
K. Abdhul, M. Ganesh, S. Shanmughapriya, M. Kanagavel, K. Anbarasu, K. Natarajaseenivasan, Antioxidant activity of exopolysaccharide from probiotic strain Enterococcus faecium (BDU7) from Ngari. Int. J. Biol. Macromol. 70, 450–454 (2014)
A. Sohail, M.S. Turner, A. Coombes, T. Bostrom, B. Bhandari, Survivability of probiotics encapsulated in alginate gel microbeads using a novel impinging aerosols method. Int. J. Food Microbiol. 145, 162–168 (2011)
D. Guerin, J.C. Vuillemard, M. Subirade, Protection of bifidobacteria encapsulated in polysaccharide-protein gel beads against gastric juice and bile. J. Food Prot. 66, 2076–2084 (2003)
K. Feng, R.M. Huang, R.Q. Wu, Y.S. Wei, M.H. Zong, R.J. Linhardt, H. Wu, A novel route for double-layered encapsulation of probiotics with improved viability under adverse conditions. Food Chem. 310, 125977 (2020)
M. Afzaal, F. Saeed, M.U. Arshad, M.T. Nadeem, M. Saeed, T. Tufail, The effect of encapsulation on the stability of probiotic bacteria in ice cream and simulated gastrointestinal conditions. Probiotics Antimicrob Proteins. 11, 1348–1354 (2019)
W.K. Ding, N.P. Shah, Effect of various encapsulating materials on the stability of probiotic bacteria. J. Food Sci. 74, 100–107 (2009)
A.B. Shori, Microencapsulation improved probiotics survival during gastric transit. HAYATI J. Biosci. 24, 1–5 (2017)
P. Darjani, M.H. Nezhad, R. Kadkhodaee, E. Milani, Influence of prebiotic and coating materials on morphology and survival of a probiotic strain of Lactobacillus casei exposed to simulated gastrointestinal conditions. LWT Food Sci. Technol. 73, 162–167 (2016)
Q. Zou, J. Zhao, X. Liu, F. Tian, H. Zhang, H. Zhang, W. Chen, Microencapsulation of Bifidobacterium bifidum F-35 in reinforced alginate microspheres prepared by emulsification/internal gelation. Int. J. Food Sci. Technol. 46, 1672–1678 (2011)
B. Ismail, K.M. Nampoothiri, Exopolysaccharide production and prevention of syneresis in starch using encapsulated probiotic Lactobacillus plantarum. Food Technol. Biotechnol. 48, 484–489 (2010)
M. Afzaal, F. Saeed, H. Ateeq, A. Ahmed, A. Ahmad, T. Tufail, Z. Ismail, F.M. Anjum, Encapsulation of Bifidobacterium bifidum by internal gelation method to access the viability in cheddar cheese and under simulated gastrointestinal conditions. Food Sci. Nutr. 8, 1–9 (2020)
C. González-Ferrero, J.M. Irache, B. Marín-Calvo, L. Ortiz-Romero, R. Virto-Resano, C.J. González-Navarro, Encapsulation of probiotics in soybean protein-based microparticles preserves viable cell concentration in foods all along the production and storage processes. J. Microencapsul. 37, 242–253 (2020)
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no relevant conflict of interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Abedfar, A., Hosseininezhad, M., Sadeghi, A. et al. Comparative survival of exopolysaccharide encapsulated Lactobacillus plantarum and Pediococcus pentosaceus in simulated gastrointenstinal conditions and storage time. Food Measure 15, 594–603 (2021). https://doi.org/10.1007/s11694-020-00664-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11694-020-00664-1