Abstract
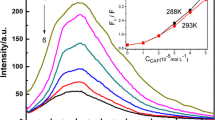
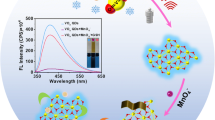
A new method for the determination of glutathione was established based on the principle of fluorescence quenching. The reaction mechanism has been studied by the measurement of fluorescence lifetime and based on the Stern–Volmer plot. The binding constant, K = 9.86 × 105 J mol−1 and the number of binding sites n = 1.12 were obtained against this reaction. The thermodynamic parameters were estimated. The data, ΔG = −34.19 KJ mol−1, ΔH = −182.2 KJ mol−1, and ΔS = −496.8 J K−1 mol−1 showed that the reaction was spontaneous and exothermic. The calibration curve was found to be linear between the fluorescence quenching (F 0 /F) and the concentration of glutathione with the range of 3.0 × 10−5–3.6 × 10−3 g/L. The detection limit was 4.5 μg/L and the relative standard derivative was 3.32% for 11 replicate determination of 6.0 × 10−4 g/L glutathione. This method can be used for the determination of glutathione in fresh vegetables with satisfactory results.








Similar content being viewed by others
References
P. Monostori, G. Wittmann, E. Karg, S. Turi, Deternination of glutathione and glutathione disulfide in biological sample: an in-depth review. J. Chromatogr. B 877, 3331–3346 (2009)
K.C. Sekhar, R. Syed, M. Golla, Novel heteroaryl phosphonicdiamides PTPs inhibitors as anti-hyperglycemic agents. DARU J. Pharm. Sci. 22, 76 (2014)
I. Rahaman, A. Kode, S.K. Biswas, Assay for quantitative determination of glutathione and glutathione disulfide levels using. Nat. Protoc. 1, 3159–3165 (2006)
A. Musenga, R. Mandrioli, P. Bonifazi, Sensitive and selective determination of glutathioine in probiotic bacteria by capillary electrophotesis-laser induced fluotescence. Anal. Bioanal. Chem. 387, 917–924 (2007)
D. Giustarini, P. Fanti, E. Matteucci, R. Rossi, Micro-method for the determination of glutathione in human blood. J. Chromatogr. B 964, 191–194 (2014)
Y.H. Chen, F.S. Tian, R. Jiang, Spectrophotometric determination of glutathione based inhibitory effect on hemoglobin. Asian J. Chem. 22, 6007–6012 (2010)
J.P. Rafael, V. Antonio, S. Manuel, P.B. Dolores, Automatic kinetic method for the determination of reduced glutathione in blood. Anal. Chim. Acta 269, 273–279 (1992)
Y.H. Chen, R.X. Cai, Highly sensitive fluorimetic determination of glutathione based inhibitory effect on multienzyme redox system. Spectrochim. Acta Part A 61, 3051–3055 (2005)
J.C. Harfield, C. Batchelor-McAuley, R.G. Compton, Electrochemical determination of glutathione: a review. Analyst. 137, 2285–2296 (2012)
J.G. Wang, H. Lü, Q.H. Sun, Method of electrochemical determination of glutathione. Acta Chim. Sin. 67, 415–419 (2009)
J.B. Raoof, R. Ojani, M. Kolbadinezhad, Voltammetric sensor for glutathione determination based on ferrocene-modified carbon paste electrode. J. Solid State Electrochem. 13, 1411–1416 (2009)
X.C. Guo, P. Xie, J. Chen, X. Tuo, X.W. Deng, S.C. Li, D.Z. Yu, C. Zeng, Simultaneous quantitative determination of microcystin-LR and its glutathione metabolites in rat liver by liquid chromatography–tandem mass spectrometry. J. Chromatogr. B 963, 54–61 (2014)
M. Dai, P. Xie, G.D. Liang, Simultaneous determination of microcystin-LR and its glutathione conjugate in fish tissues by liquid chromatography-tandem mass spectrometry. J. Chromatogr. B 862, 43–50 (2008)
L.Y. Zhang, M.X. Sun, Fast determination of glutathione by capillary electrophoresis with fluorescence detection using β-cyclodextrin as modifier. J. Chromatogr. B 877, 4051–4054 (2009)
W. Wang, H. Xin, H.L. Shao, W.R. Jin, Determination of glutathione in single human hepatocarcinoma cells by capillary electrophoresis with electrochemical detection. J. Chromatogr. B 889, 425–429 (2003)
E.H. Hansen, Flow-injection enzymatic assays. Anal. Chim. Acta 216, 257–273 (1989)
M. Sono, M.P. Roach, E.D. Coulter, Heme-containing oxygenases. Chem. Rev. 96, 2841–2887 (1996)
Y. Saito, M. Mifune, S.J. Nakashima, Determination of hydrogen peroxide with N,N-diethylaniline and 4-aminoantipyrine by use of an anion-exchange resin modified with managanese tetrakis(sulfopheny) porphine as a substitute for peroxidase. Talanta 34, 667–669 (1987)
Y.X. Ci, F. Wang, Mimesis of peroxidase by Mn-TMPyPin in the catalytic fluorescence peaction of the homovanillic acid-hydrogen peroxide system. Mikrochim. Acta 100, 63–68 (1990)
Q.Z. Zhu, Q.G. Li, J.Z. Lu, Application of thiamine as afluorogenic substrate in the determination of hydrogen peroxidebased on the catalytic effect of hemin. Anal. Lett. 29, 1729–1740 (1996)
L.Y. Mao, M. Zhu, H.X. Shen, Study on immobilized supramolecular inclusion compled of iron-porphyrin as an analogue for peroxide proteinase. Chem. J. Chin. Univ. 19, 442–445 (1998)
G.B. Jameson, F.S. Molinaro, J.I. Brauman, Models for the active site of oxygen-binding hemoproteins. Dioxygen binding properties and the structures of (2-methylimidazole)- meso-tetra(.alpha.,.alpha.,.alpha.,.alpha.-o-pivalamidophenyl)porphyrinatoiron(II)-ethanol and its dioxygen adduct. J. Am. Chem. Soc. 102, 3224–3237 (1980)
T. Brittain, Molecular aspects of embryonic hemoglobin function. Mol. Aspects. Med. 23, 293–342 (2002)
T.M. Larsen, T.C. Mueser, L.J. Parkhurst, Use of dual wavelength spectrophotometry and continuous enzymatic depletion of oxygen for determination of the oxygen binding constants of hemoglobin. Anal. Biochem. 197, 231–246 (1991)
K. Zhang, R. Cai, D. Chen, Determination of hemoglobin based on its enzymatic activity for the oxidation of o-phenylenediamine with hydrogen peroxide. Anal. Chim. Acta 413, 109–113 (2000)
Y. Chen, F. Tian, G. Zhang, High-sensitivity spectrofluorimetric determination of tiopronin based on inhibition of hemoglobin. Luminescence 26, 477–480 (2011)
J.R. Lakowicz, Principles of Fluorescence Spectroscopy, 2nd edn. (Plenum Press, New York, 1986), p. 264
G.Z. Chen, X.Z. Huang, Z.Z. Zheng, Method of Fluorescence Analysis, 2nd edn. (Science Press, Beijing, 1991), pp. 115–116
M. Alain, B. Michel, D. Michel, How to illustrate ligand-protein binding in a class experiment: an elementary fluorescent assay. J. Chem. Educ. 63, 365–366 (1986)
Q.Y. Xing, R.Q. Xu, Z. Zhou, The Fundament of Organic Chemistry, 2nd edn (Science Press, Beijing, 1993), p. 454
M. Zhang, Q.L. Lv, N.N. Yue, H.Y. Wang, Study of fluorescence quenching mechanism between quercetin and tyrosine-H2O2-enzyme catalyzed product. Spectrochim. Acta A 72, 572–576 (2009)
P.D. Ross, S. Subramanian, Thermodynamics of protein association reactions: forces contributing to stability. Biochemistry 20, 3096–3102 (1981)
R. Gotti, V. Andrisano, V. Cavrini, A. Bongini, Determination of glutathione in pharmaceuticals and cosmetics by HPLC with UV and fluorescence detection. Chromatography 39, 23–28 (1994)
Acknowledgements
This work was sponsored by the Natural Science Foundation of Henan Province of China (No. 172102310626) and supported by the Natural Science Foundation of Education Department of Henan Province of China (No. 16B416001).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Chen, Y., Wang, Q., Liu, L. et al. Fluorescence quenching and measurement of glutathione in fresh vegetables. Food Measure 12, 221–227 (2018). https://doi.org/10.1007/s11694-017-9633-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11694-017-9633-z