Abstract
The ability of wildlife to endure the effects of high temperatures is increasingly important for biodiversity conservation under climate change and spreading urbanization. Organisms living in urban heat islands can have elevated heat tolerance via phenotypic or transgenerational plasticity or microevolution. However, the prevalence and mechanisms of such thermal adaptations are barely known in aquatic organisms. Furthermore, males and females can differ in heat tolerance, which may lead to sex-biased mortality, yet it is unknown how sex differences in thermal biology influence urban phenotypic divergence. To address these knowledge gaps, we measured critical thermal maxima (CTmax) in male and female agile frog (Rana dalmatina) tadpoles captured from warm urban ponds and cool woodland ponds, and in a common-garden experiment where embryos collected from both habitat types were raised in the laboratory. We found higher CTmax in urban-dwelling tadpoles compared to their counterparts living in woodland ponds. This difference was reversed in the common-garden experiment: tadpoles originating from urban ponds had lower CTmax than tadpoles originating from woodland ponds. We found no effect of sex on CTmax or its difference between habitats. These results demonstrate that aquatic amphibian larvae can respond to the urban heat island effect with increased heat tolerance similarly to other, mostly terrestrial taxa studied so far, and that phenotypic plasticity may be the main driver of this response. Our findings also suggest that heat-induced mortality may be independent of sex in tadpoles, but research is needed in many more taxa to explore potentially sex-dependent urban thermal responses.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Heat tolerance, i.e. the capacity to cope with high temperatures, is becoming increasingly important throughout the tree of life with ongoing climate change. In many regions across the globe, not only are average temperatures rising but heat events are also getting more frequent (Perkins-Kirkpatrick & Lewis, 2020). High temperature can be directly lethal by physiological breakdown due to heat stress, but it may also cause secondary mortality by making organisms more susceptible to predation or disease (Kroeker & Sanford, 2022). Populations can better tolerate high temperatures by two mutually non-exclusive mechanisms: phenotypic plasticity expressed during the lifetime of individuals (acclimation or thermal plasticity), or over generations via microevolution, epigenetic modifications or other forms of transgenerational plasticity (Lambert et al., 2021; Urban et al., 2014). Mechanisms matter because phenotypically plastic responses can manifest much faster but can also be costly and their scope may be constrained (Gunderson & Stillman, 2015; Murren et al., 2015; Radchuk et al., 2019).
Dealing with heat stress is especially relevant for organisms living in urbanized habitats, because heat storage in buildings and sealed roads makes cities warmer compared to surrounding non-urban areas, and this urban heat island effect is amplified during heat waves (Li & Bou-Zeid, 2013). Accordingly, it has been shown in a variety of ectothermic species that urban populations have higher heat tolerance, expressed as the critical thermal maximum (CTmax), the upper temperature at which animals lose the ability to function (Diamond & Martin, 2021). These phenotypic changes can be adaptive (Brans et al., 2017; Martin et al., 2021) and result from a combination of phenotypic plasticity and local adaptation by microevolution (Brans et al., 2017; Diamond et al., 2017).
Heat tolerance may also differ between sexes. For example, female mice and male salmon tolerate heat better than their opposite-sex conspecifics (Garcia et al., 2018; Jeffries et al., 2012). This has important implications for evolutionary ecology and conservation biology. Sex-dependent mortality can lead to skewed sex ratios, which then constrain effective population size and adaptive potential but can also catalyze evolutionary changes in sex-specific life histories and social systems (Mitchell & Janzen, 2010; Schacht et al., 2022). Also, skewed sex ratios can have cascading effects on other species and even ecosystems (Edmands, 2021). Despite this significance of the issue, there is a dearth of information on sex differences in heat tolerance (Edmands, 2021; Pottier et al., 2021). Furthermore, sexes may also differ in their capacity for thermal plasticity (Pottier et al., 2021), yet next to nothing is known about the role of sex in thermal responses to urban heat islands.
For several organismal traits such as hormone levels, cognitive performance, and parasite load, it has been demonstrated that urbanization can have sex-specific effects (Bonier et al., 2007; Preiszner et al., 2017; Sykes et al., 2021). Because males and females can differ in the temperatures they experience, prefer, or tolerate due to differences in size, life history, and behaviors associated with reproduction (Edmands, 2021; Ruckstuhl & Neuhaus, 2005), the sexes may also differ in the selection pressures that urban heat islands exert on them. Then, sex-dependent selection for thermal tolerance and/or for acclimation capacity may complicate thermal adaptation of urban populations due to genetic correlations between the sexes and sexual selection (Leith et al., 2022). However, our understanding of these potential outcomes is highly deficient due to the virtual lack of empirical studies on sex differences in the effects of urbanization on heat tolerance.
Although the number of studies published on urban heat tolerance is increasing exponentially (Roeder et al., 2021), almost all this effort has been focused on terrestrial organisms (Diamond & Martin, 2021). Aquatic ectotherms, however, are also exposed to the urban heat island effect (Brans et al., 2018) and may be especially vulnerable to it due to limited dispersal (Pagliaro & Knouft, 2020) and the conflict between increased demand for and decreased supply of oxygen in warmer water (Brans et al., 2017). Here, we investigated heat tolerance in tadpoles of the agile frog (Rana dalmatina), a European species with decreasing population trends that occurs in both urban and non-urban habitats (Kaya et al., 2009). First, we show that individuals from relatively warm urban ponds and those from relatively cool non-urban ponds differ in heat tolerance measured as CTmax. Second, we report a common garden experiment to infer whether this difference is attributable to individual plasticity or transgenerational change (including microevolution and/or transgenerational plasticity). Finally, we compare heat tolerance between males and females and test whether the effect of urbanization on heat tolerance is sex-dependent.
Methods
Pond Temperatures
We used six study sites: three were in hilly woodlands with < 0.1% anthropogenically modified habitat within 500 m of each pond, whereas the other three sites were in three different townships with ca. 70% anthropogenically modified land cover within 500 m of the ponds (Table S1). The chosen ponds were representative of their respective habitat type based on land-use metrics and preliminary temperature measurements taken in the previous year. In each pond, we recorded water temperature every 30 min from 6th April to 8th July 2022 using Onset UA-002-64 HOBO loggers. We placed four loggers within each pond in the area where we collected eggs (see below). One pair of loggers was placed in the deepest water we could access, whereas another pair of loggers was placed close to the shore, at ≤ 30 cm water depth. Within each pair, one logger was ca. 5 cm under the water surface and one was ca. 5 cm above the bottom. We monitored the loggers weekly and adjusted their position to follow changes in water depth (for further information see Fig. S1).
Experimental Protocol
From the six study ponds, we collected three cohorts of animals. For the first two cohorts, we collected freshly spawned agile frog eggs on 4th April, and 3-weeks old embryos (right before hatching) on 20th April. From each pond, we took ca. 20 embryos from each of four egg masses for each of the two cohorts (Table S1) and transported them to our laboratory. We kept each sibling group in a separate container with ca. 1 cm deep reconstituted soft water (RSW; 48 mg NaHCO3, 30 mg CaSO4 × 2 H2O, 61 mg MgSO4 × 7 H2O, 2 mg KCl added to 1 L reverse-osmosis filtered, UV-sterilized, aerated tap water). Over the course of the study, temperature in the lab was set to gradually increase from 18 to 20 °C (mean ± standard deviation: 19.3 ± 0.9 °C) and we regularly adjusted the photoperiod to mimic the natural dark–light cycles. When the animals reached the free-swimming state, i.e. developmental stage 25 according to (Gosner, 1960), we placed them individually in 2-L plastic rearing containers filled with 1 L RSW, arranged in a randomized block design to ensure that both cohorts and all six populations were homogeneously distributed across the shelves in the laboratory. We changed the rearing water twice a week and fed the tadpoles ad libitum with chopped, slightly boiled spinach. On the 18th–20th day after reaching developmental stage 25, we randomly selected six tadpoles from each sibling group (Table S1), resulting in a total sample size of 144 tadpoles (24 per pond) from each cohort, and tested their CTmax (see below). The remaining tadpoles were released at their ponds of origin.
From the same six ponds, we collected the third cohort as tadpoles by dip-netting in the second half of May, when the animals were at a similar developmental stage (having only small hindlimb buds) as the captive-reared tadpoles were at the time of CTmax testing. We aimed to collect 24 tadpoles from each pond, but we did not find any in one of the urban ponds and we could capture only six from a woodland (non-urban) pond, yielding a total sample size of 102 free-living tadpoles (Table S1). We transported the tadpoles to our laboratory, housed and fed them the same way as the captive-reared tadpoles, and tested their CTmax one or two days after their collection.
We measured CTmax in a randomized order within each cohort by placing 8 tadpoles, each in its original rearing container, into a tray (80 × 60 × 12 cm) in which water was heated with two digital thermostat heaters (BRH Heizung LCD Turbo 600) and circulated by two water pumps (Tetra WP 300). For the time of the test, each tadpole’s container was filled with 1.5 L fresh RSW, and when all 8 containers were placed in the tray, water level in the tray was ca. 0.5 cm below that in the tadpole containers. We increased water temperature in the tadpoles’ containers at a rate of 0.6 °C/min. Twenty-one minutes after placing the containers into the tray, we started to observe the animals, lightly prodding the base of their tail every 6 s. We defined CTmax as the temperature (as measured by Greisinger digital thermometers GTH175/PT; ± 0.1 °C) at which the tadpole failed to respond with motion over three consecutive prods. The test was performed by six experimenters, four at a time, each person overseeing two tadpoles always at the same two positions within the tray.
After the CTmax test, we weighed each tadpole (± 0.1 mg), and recorded its developmental stage by stereomicroscopic examination. Animals that survived the CTmax test were euthanized in a water bath of 6 g/L tricaine-methanesulfonate (MS-222) buffered to neutral pH with the same amount of disodium hydrogen phosphate. To preserve DNA for genetic sexing, we stored every tadpole in 96% ethanol. We extracted DNA using E.Z.N.A. Tissue DNA Kit following the manufacturer’s protocol, except that digestion time was at least 3 hours. For genetic sexing, we used the method of Nemesházi et al. (2020). Briefly, we tested all tadpoles for sex marker Rds3 (≥ 95% sex linkage; primers: Rds3-HRM-F and Rds3-HRM-R) using high-resolution melting (Fig. S2). The total HRM reaction volume was 15 µL, containing 7.5 µL 2x PerfeCTa® SYBR® Green SuperMixes (ROX, Quantabio), 1 µL forward and 1 µL reverse primer (10 µM each), and 80–100 ng genomic DNA in MQ water to reach the final volume. Reactions were performed in a Quantabio Q 4-channel qPCR Instrument and the results were analysed with the 1.0.2. version Q-qPCR Software (Quantabio).
Statistical Analyses
We used R 4.2.2 for all analyses (R Core Team, 2022). We analyzed pond temperatures using a generalized additive mixed model (‘gamm’ function of package ‘mgcv), because the change of temperature over time was not linear (Fig. S1). We included pond identity and logger position as fixed factors, and time as a covariate and temporal autocorrelation (order-1 auto-regressive model) within the data of each logger. For comparison among the ponds, we extracted marginal means for each pond from the model and calculated linear contrasts pairwise and also between the three urban and three woodland ponds (‘emmeans’ function of package ‘emmeans’). For the pairwise comparisons, we corrected the P-values with the false discovery rate (FDR) method (Pike, 2011).
To analyze CTmax, we used a generalized estimation equations (GEE) model (‘geeglm’ function of package ‘geepack’). GEE is a population-averaging method that can handle the correlation structure of our data (i.e. tadpoles from the same pond are not independent, but the pond effect is nested within the habitat effect) appropriately and without penalizing power (Zuur et al., 2009). We included the following fixed factors: habitat type (urban or woodland), cohort (collected as eggs, embryos, or tadpoles), sex, their three-way and all two-way interactions, body mass, developmental stage, and experimenter identity; we used pond of origin as a random factor (‘compound symmetry’ correlation structure). From this initial full model, we evaluated the significance of interactions by analysis-of-deviance tables. Then we dropped the non-significant interactions stepwise, to facilitate the accuracy and ease of estimation for the significant interaction term. We calculated linear contrasts (as above, correcting the P-values with the FDR method) to test the habitat effect within each cohort from the final GEE model that contained only significant interactions but all main effects regardless of their significance.
Results
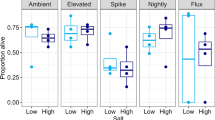
All urban ponds were significantly warmer than all woodland ponds (Figs. S1 and S3), on average by 5.27 °C ± 0.18 SE (urban-woodland contrast, P < 0.001). For CTmax, the three-way interaction between sex, cohort, and habitat of origin was non-significant (χ22 = 2.70, P = 0.259; Fig. S4), and so were the two-way interactions between sex and cohort (χ22 = 1.38, P = 0.501) and between sex and habitat of origin (χ21 = 0.13, P = 0.713). According to the final model, females had slightly higher CTmax than males (by 0.07 ± 0.04 °C; Fig. 1), but this difference was not statistically significant, although not far from the 5% significance threshold (P = 0.090). The interaction between cohort and habitat of origin was highly significant (χ22 = 58.4, P < 0.001; Fig. 1), such that tadpoles living in urban habitats had higher CTmax than tadpoles living in woodland habitats (by 0.15 ± 0.05 °C, P = 0.004), but this difference was reversed when the animals were raised in a common, captive environment after being collected from the field as freshly spawned eggs (by 0.17 ± 0.09 °C, P = 0.045) or three-weeks old embryos (by 0.20 ± 0.08 °C, P = 0.016).
Critical thermal maximum (CTmax) of agile frog tadpoles originating from woodland or urban habitats and raised in the field or in captivity from the egg or embryo stage, and by sex. Means with standard errors (SE) were corrected for body mass, developmental stage, experimenter identity, and pseudoreplication (non-independence of tadpoles from the same site) using a generalized estimation equations model
Discussion
We found that urban ponds were several degrees warmer, supporting Brans et al. (2018) and Pagliaro & Knouft (2020) that urbanization is accompanied by higher temperatures of freshwater habitats in the temperate climate zone. This urban heat island effect was accompanied by a small but statistically significant increase in CTmax for agile frog tadpoles developing in urban ponds compared to their counterparts inhabiting woodland ponds. This difference is similar in magnitude to those found between cooler and warmer environments in other amphibians and to those expected based on the relatively low thermal acclimation capacity of amphibians and the slow pace of CTmax adaptation due to the “Bogert effect” (Bodensteiner et al., 2021; Skelly & Freidenburg, 2000). Our result aligns with the findings of Brans et al. (2017) on water fleas, demonstrating that aquatic organisms respond to urbanization by higher heat tolerance similarly to a wide variety of terrestrial taxa ranging from fungi through arthropods to lizards (Campbell-Staton et al., 2020; Diamond & Martin, 2021; McLean et al., 2005). This phenotypic response is important for several reasons. First, already these days, pond temperatures occasionally rise so high (Lambert et al., 2018; Lindauer et al., 2020) that they approach or exceed the upper thermal tolerance limits of aquatic animals (Brans et al., 2017; Pagliaro & Knouft, 2020), especially so in urban heat islands (see Fig. S1); and such heat events are expected to get more frequent in the future due to climate change. Second, CTmax is not a standalone physiological trait, as it may be part of a “thermal syndrome”, linked with other aspects of thermal performance and preference, and potentially also with behavioral and life-history traits (Goulet et al., 2017; Goulet et al., 2017). For example, individuals with higher CTmax can also better tolerate ecologically relevant slow-rate warming (Åsheim et al., 2020). Often, the magnitude of heat tolerance decreases with the duration of heat (Troia, 2023); for example, agile frog tadpoles can experience high mortality during a 6-days long period at 28°C (Ujszegi et al., 2022) despite their ≥ 33.6°C CTmax (according to the present study). Such a several-days heat wave has not only lethal effects but also induces sex reversal in developing larvae (Ujszegi et al., 2022) which may have wide-ranging consequences for individual fitness and population persistence (Bókony et al., 2017; Bókony et al., 2021; Nemesházi et al., 2021). Thus, a “thermal type” with elevated tolerance against the various physiological harms of high temperatures is likely to be adaptive particularly for urban populations which are more frequently exposed to heat.
This surmised adaptive response can come about through individual plasticity or by changes accumulating over the course of many generations. Our study suggests that the former mechanism is the main driver of higher CTmax in urban agile frog tadpoles, because we observed elevated heat tolerance only in the free-living urban animals and not in the urban-collected animals raised in the common-garden experiment from early or late embryonic stages. Phenotypic plasticity is further suggested by the higher CTmax of free-living tadpoles which experienced higher temperatures in the field (up to 28.46 °C before being collected for CTmax testing; Fig. S1) than did their captive-raised counterparts (up to 19.9 °C). Our study was not designed to identify the mechanism of phenotypic plasticity or to measure the contribution of short-term, reversible acclimatization and longer-lasting, more slowly establishing developmental plasticity (Beaman et al., 2016). However, CTmax of amphibians can acclimate within a few days (Hutchison & Maness, 1979; Layne & Claussen, 1982; Turriago et al., 2023), suggesting that the 1–2 days acclimation period in our study might have canceled (some of the) differences that had been induced by acclimatization to the temperatures experienced in urban and woodland ponds, leaving room for developmental plasticity to potentially manifest in our measurements.
The lack of support for local adaptation by microevolution or other transgenerational changes in tadpole CTmax is in contrast with findings on arthropods where, besides phenotypic plasticity, microevolution also proved an important driver of urban heat tolerance (Diamond & Martin, 2021). It is possible that the relatively long generation time in frogs may not have permitted enough evolutionary change to accumulate in urban populations. Despite the relatively restricted gene flow across the barriers represented by anthropogenically altered habitats (Hitchings & Beebee, 1997; Lesbarrères et al., 2006), low-resistance corridors may still have allowed for sufficient levels of migration to prevent the establishment of local adaptation (Furman et al., 2016; Neal et al., 2020). Also, phenotypic plasticity seems to be favored in amphibian physiological traits, maybe due to high spatiotemporal environmental heterogeneity within urban habitats (Bókony et al., 2019; Bókony et al., 2021), which is exemplified by the relatively large microclimatic differences between nearby ponds in the present study (e.g. two ponds in Pilisszentiván, see Fig. S1 & S3). Indeed, the magnitude of thermal pollution in urban ponds is strongly affected by local-scale variation such as the “park cooling effect” and runoff (Brans et al., 2018), which might explain why higher heat tolerance was not found in some ectotherms in broad-scale urban regions (Diamond & Martin, 2021). Naturally, heterogeneous thermal microhabitats are also present outside cities, which may select for genotypes with different thermal tolerances and thereby facilitate adaptations to urban habitats (Campbell-Staton et al., 2020). However, such standing variation in heat tolerance might be limited in the agile frog because it primarily occurs in relatively cool habitats and its genetic diversity across Europe is very low (Vences et al., 2013). Furthermore, genetic divergence might also be constrained by relatively low heritability of CTmax in ectotherms (Logan & Cox, 2020).
Interestingly, in our common-garden experiment, the lack of experiencing field temperatures was not simply accompanied by no difference in CTmax; instead, the captive-raised tadpoles originating from urban ponds had lower heat tolerance than those from woodland ponds. We can only speculate about the causes of this difference. It has been demonstrated in some anuran species, including agile frogs, that compared to conspecifics originating from more natural habitats, individuals originating from urban habitats perform poorly in various fitness-related traits when raised in captivity, including slower development, reduced growth, more frequent developmental abnormalities, and higher mortality (Bókony et al., 2018, 2023; Hitchings & Beebee, 1997). The reasons are unclear but may be related to transgenerational effects of endocrine-disrupting chemical pollutants (Bókony et al., 2018) or inbreeding in isolated urban populations due to landscape fragmentation (Hitchings & Beebee, 1997; Lesbarrères et al., 2006). Lower heat tolerance might be another manifestation of this overall poor physiological fitness; for example, due to a genetic correlation between growth rate and CTmax (Martins et al., 2019). An alternative explanation might be that the higher heat tolerance of free-living urban tadpoles was conferred by “maternal programming” which resulted in the opposite outcome in the mismatched captive environment (Monaghan, 2008). Additionally, countergradient variation and/or divergence in plastic responses (i.e. crossing reaction norms) might also underlie our findings (Bodensteiner et al., 2021; Lambert et al., 2021). To tease apart and test these alternatives, further common-garden experiments including multiple rearing temperatures will be needed.
Finally, we found no difference between male and female tadpoles in the effect of urbanization on CTmax, although there was a slight, non-significant trend for higher heat tolerance in females. In adult agile frogs, the two sexes may face different selection pressures on thermal tolerance for several reasons. For example, females typically mature at larger body sizes than males, which may affect multiple aspects of their thermal physiology (Rohr et al., 2018; Rubalcaba et al., 2019). Furthermore, males migrate earlier from hibernacula to the breeding ponds, sometimes even when snow and ice have not yet melted (Riis, 1997), whereas females were reported to forage less in open microhabitats compared to males (Cicort-Lucaciu et al., 2011). However, even if these microclimatic differences select for different thermal physiology in the two sexes, it is possible that those differences may not become expressed until sexual maturity, as sex seems to have little effect on behavior and life history in immature agile frogs (Bókony et al., 2021). More studies are needed, ideally with multiple age groups and life-history stages, to increase our knowledge from next to nothing about sex differences in temperature sensitivities of amphibians and various other taxa (Edmands, 2021). That knowledge will be necessary for inferring the role of sex-biased mortality via sex-dependent thermal tolerance in the effects of urbanization and climate change on the fate of wildlife populations and biodiversity.
Data Availability
All data analyzed in this paper are available as online supplementary material.
References
Åsheim, E., Andreassen, A., Morgan, R., & Jutfelt, F. (2020). Rapid-warming tolerance correlates with tolerance to slow warming but not growth at non-optimal temperatures in zebrafish. Journal of Experimental Biology, 223(23), jeb229195. https://doi.org/10.1242/jeb.229195.
Beaman, J. E., White, C. R., & Seebacher, F. (2016). Evolution of plasticity: mechanistic link between development and reversible acclimation. Trends in Ecology and Evolution, 31(3), 237–249. https://doi.org/10.1016/j.tree.2016.01.004.
Bodensteiner, B. L., Agudelo-Cantero, G. A., Arietta, A. Z. A., Gunderson, A. R., Muñoz, M. M., Refsnider, J. M., & Gangloff, E. J. (2021). Thermal adaptation revisited: How conserved are thermal traits of reptiles and amphibians? Journal of Experimental Zoology Part A: Ecological and Integrative Physiology, 335(1), 173–194. https://doi.org/10.1002/jez.2414.
Bókony, V., Kalina, C., Ujhegyi, N., Mikó, Z., Lefler, K. K., Vili, N., et al. (2023). Does stress make males? An experiment on the role of glucocorticoids in anuran sex reversal. bioRxiv. https://doi.org/10.1101/2023.05.27.541969.
Bókony, V., Pipoly, I., Szabó, K., Preiszner, B., Vincze, E., Papp, S., et al. (2017). Innovative females are more promiscuous in great tits (Parus major). Behavioral Ecology, 28(2), 579–588. https://doi.org/10.1093/beheco/arx001.
Bókony, V., Ujhegyi, N., Hamow, K., Bosch, J., Thumsová, B., Vörös, J., et al. (2021). Stressed tadpoles mount more efficient glucocorticoid negative feedback in anthropogenic habitats due to phenotypic plasticity. Science of the Total Environment, 753, 141896. https://doi.org/10.1016/J.SCITOTENV.2020.141896
Bókony, V., Ujhegyi, N., Mikó, Z., Erös, R., Hettyey, A., Vili, N., et al. (2021). Sex reversal and performance in fitness-related traits during early life in agile frogs. Frontiers in Ecology and Evolution, 9, 745752. https://doi.org/10.3389/fevo.2021.745752.
Bókony, V., Üveges, B., Ujhegyi, N., Verebélyi, V., Nemesházi, E., Csíkvári, O., & Hettyey, A. (2018). Endocrine disruptors in breeding ponds and reproductive health of toads in agricultural, urban and natural landscapes. Science of the Total Environment, 634, 1335–1345. https://doi.org/10.1016/j.scitotenv.2018.03.363
Bókony, V., Üveges, B., Verebélyi, V., Ujhegyi, N., & Móricz, Á. M. (2019). Toads phenotypically adjust their chemical defences to anthropogenic habitat change. Scientific Reports, 9, 3163. https://doi.org/10.1038/s41598-019-39587-3.
Bonier, F., Martin, P. R., Sheldon, K. S., Jensen, J. P., Foltz, S. L., & Wingfield, J. C. (2007). Sex-specific consequences of life in the city. Behavioral Ecology, 18(1), 121–129. https://doi.org/10.1093/beheco/arl050.
Brans, K. I., Engelen, J. M. T., Souffreau, C., & De Meester, L. (2018). Urban hot-tubs: Local urbanization has profound effects on average and extreme temperatures in ponds. Landscape and Urban Planning, 176, 22–29. https://doi.org/10.1016/j.landurbplan.2018.03.013.
Brans, K. I., Jansen, M., Vanoverbeke, J., Tüzün, N., Stoks, R., & De Meester, L. (2017). The heat is on: Genetic adaptation to urbanization mediated by thermal tolerance and body size. Global Change Biology, 23(12), 5218–5227. https://doi.org/10.1111/gcb.13784.
Campbell-Staton, S. C., Winchell, K. M., Rochette, N. C., Fredette, J., Maayan, I., Schweizer, R. M., & Catchen, J. (2020). Parallel selection on thermal physiology facilitates repeated adaptation of city lizards to urban heat islands. Nature Ecology and Evolution, 4(4), 652–658. https://doi.org/10.1038/s41559-020-1131-8.
Cicort-Lucaciu, A. S., Sas, I., Roxin, M., Badar, L., & Goilean, C. (2011). The feeding study of a Rana dalmatina population from Carei Plain. South Western Journal of Horticulture Biology and Environment, 2(1), 35–46.
Diamond, S. E., & Martin, R. A. (2021). Physiological adaptation to cities as a proxy to forecast global-scale responses to climate change. Journal of Experimental Biology, 224, jeb229336. https://doi.org/10.1242/jeb.229336.
Diamond, S. E., Chick, L., Perez, A., Strickler, S. A., & Martin, R. A. (2017). Rapid evolution of ant thermal tolerance across an urban-rural temperature cline. Biological Journal of the Linnean Society, 121(2), 248–257. https://doi.org/10.1093/BIOLINNEAN/BLW047.
Edmands, S. (2021). Sex ratios in a warming world: thermal effects on sex-biased survival, sex determination, and sex reversal. Journal of Heredity, 112(2), 155–164. https://doi.org/10.1093/jhered/esab006.
Furman, B. L. S., Scheffers, B. R., Taylor, M., Davis, C., & Paszkowski, C. A. (2016). Limited genetic structure in a wood frog (Lithobates sylvaticus) population in an urban landscape inhabiting natural and constructed wetlands. Conservation Genetics, 17(1), 19–30. https://doi.org/10.1007/s10592-015-0757-6.
Garcia, C. K., Mattingly, A. J., Robinson, G. P., Laitano, O., King, M. A., Dineen, S. M., et al. (2018). Sex-dependent responses to exertional heat stroke in mice. Journal of Applied Physiology, 125(3), 841–849. https://doi.org/10.1152/japplphysiol.00220.2018.
Gosner, K. L. (1960). A simplified table for staging anuran embryos and larvae with notes on identification. Herpetologica, 16(3), 183–190. https://doi.org/10.2307/3890061.
Goulet, C. T., Thompson, M. B., Michelangeli, M., Wong, B. M., & Chappele, D. G. (2017). Thermal physiology: A new dimension of the pace-of‐life syndrome. Journal of Animal Ecology, 86, 1269–1280. https://doi.org/10.1111/1365-2656.12718.
Goulet, C. T., Thompson, M. B., & Chapple, D. G. (2017). Repeatability and correlation of physiological traits: Do ectotherms have a thermal type? Ecology and Evolution, 7(2), 710–719. https://doi.org/10.1002/ece3.2632.
Gunderson, A. R., & Stillman, J. H. (2015). Plasticity in thermal tolerance has limited potential to buffer ectotherms from global warming. Proceedings of the Royal Society B: Biological Sciences, 282(1808). https://doi.org/10.1098/rspb.2015.0401
Hitchings, S. P., & Beebee, T. J. C. (1997). Genetic substructuring as a result of barriers to gene flow in urban Rana temporaria (common frog) populations: Implications for biodiversity conservation. Heredity, 79(2), 117–127. https://doi.org/10.1038/hdy.1997.134.
Hutchison, V. H., & Maness, J. D. (1979). The role of behavior in temperature acclimation and tolerance in ectotherms. Integrative and Comparative Biology, 19(1), 367–384. https://doi.org/10.1093/icb/19.1.367.
Jeffries, K. M., Hinch, S. G., Martins, E. G., Clark, T. D., Lotto, A. G., Patterson, D. A., et al. (2012). Sex and proximity to reproductive maturity influence the survival, final maturation, and blood physiology of pacific salmon when exposed to high temperature during a simulated migration. Physiological and Biochemical Zoology, 85(1), 62–73. https://doi.org/10.1086/663770.
Kaya, U., Kuzmin, S., Sparreboom, M., Ugurtas, I. H., Tarkhnishvili, D., Anderson, S. (2009). Rana dalmatina. The IUCN Red List of Threatened Species, e.T58584A11790570. https://doi.org/10.2305/IUCN.UK.2009.RLTS.T58584A11790570.en.
Kroeker, K. J., & Sanford, E. (2022). Ecological leverage points: species interactions amplify the physiological effects of global environmental change in the ocean. Annual Review of Marine Science, 14, 75–103. https://doi.org/10.1146/annurev-marine-042021-051211.
Lambert, M. R., Brans, K. I., Roches, D., Donihue, S., C. M., & Diamond, S. E. (2021). Adaptive evolution in cities: progress and misconceptions. Trends in Ecology and Evolution, 36(3), 239–257. https://doi.org/10.1016/j.tree.2020.11.002.
Lambert, M. R., Smylie, M. S., Roman, A. J., Freidenburg, L. K., & Skelly, D. K. (2018). Sexual and somatic development of wood frog tadpoles along a thermal gradient. Journal of Experimental Zoology Part A: Ecological and Integrative Physiology, 329(2), 72–79. https://doi.org/10.1002/jez.2172.
Layne, J. R., & Claussen, D. L. (1982). The time courses of CTMax and CTMin acclimation in the salamander Desmognathus fuscus. Journal of Thermal Biology, 7, 139–141.
Leith, N. T., Fowler-Finn, K. D., & Moore, M. P. (2022). Evolutionary interactions between thermal ecology and sexual selection. Ecology Letters, 25(9), 1919–1936. https://doi.org/10.1111/ELE.14072.
Lesbarrères, D., Primmer, C. R., Lodé, T., & Merilä, J. (2006). The effects of 20 years of highway presence on the genetic structure of Rana dalmatina populations. Ecoscience, 13(4), 531–538. https://doi.org/10.2980/1195-6860(2006)13[531:TEOYOH]2.0.CO;2.
Li, D., & Bou-Zeid, E. (2013). Synergistic interactions between urban heat islands and heat waves: The impact in cities is larger than the sum of its parts. Journal of Applied Meteorology and Climatology, 52(9), 2051–2064. https://doi.org/10.1175/JAMC-D-13-02.1.
Lindauer, A. L., Maier, P. A., & Voyles, J. (2020). Daily fluctuating temperatures decrease growth and reproduction rate of a lethal amphibian fungal pathogen in culture. BMC Ecology, 20(1), 18. https://doi.org/10.1186/s12898-020-00286-7.
Logan, M. L., & Cox, C. L. (2020). Genetic constraints, transcriptome plasticity, and the evolutionary response to climate change. Frontiers in Genetics, 11, 538226. https://doi.org/10.3389/fgene.2020.538226.
Martin, R. A., Chick, L. D., Garvin, M. L., & Diamond, S. E. (2021). In a nutshell, a reciprocal transplant experiment reveals local adaptation and fitness trade-offs in response to urban evolution in an acorn-dwelling ant. Evolution, 75(4), 876–887. https://doi.org/10.1111/evo.14191.
Martins, F., Kruuk, L., Llewelyn, J., Moritz, C., & Phillips, B. (2019). Heritability of climate-relevant traits in a rainforest skink. Heredity, 122(1), 41–52. https://doi.org/10.1038/s41437-018-0085-y.
McLean, M. A., Angilletta, M. J., & Williams, K. S. (2005). If you can’t stand the heat, stay out of the city: Thermal reaction norms of chitinolytic fungi in an urban heat island. Journal of Thermal Biology, 30(5), 384–391. https://doi.org/10.1016/J.JTHERBIO.2005.03.002.
Mitchell, N. J., & Janzen, F. J. (2010). Temperature-dependent sex determination and contemporary climate change. Sexual Development, 4(1–2), 129–140. https://doi.org/10.1159/000282494.
Monaghan, P. (2008). Early growth conditions, phenotypic development and environmental change. Philosophical Transactions of the Royal Society B: Biological Sciences, 363(1497), 1635–1645. https://doi.org/10.1098/rstb.2007.0011.
Murren, C. J., Auld, J. R., Callahan, H., Ghalambor, C. K., Handelsman, C. A., Heskel, M. A., et al. (2015). Constraints on the evolution of phenotypic plasticity: Limits and costs of phenotype and plasticity. Heredity, 115(4), 293–301. https://doi.org/10.1038/hdy.2015.8.
Neal, K. M., Fisher, R. N., Mitrovich, M. J., & Shaffer, H. B. (2020). Conservation genomics of the threatened Western spadefoot, Spea hammondii, in urbanized Southern California. Journal of Heredity, 111(7), 613–627. https://doi.org/10.1093/jhered/esaa049.
Nemesházi, E., Gál, Z., Ujhegyi, N., Verebélyi, V., Mikó, Z., Üveges, B., et al. (2020). Novel genetic sex markers reveal high frequency of sex reversal in wild populations of the agile frog (Rana dalmatina) associated with anthropogenic land use. Molecular Ecology, 29, 3607–3621. https://doi.org/10.1111/mec.15596.
Nemesházi, E., Kövér, S., & Bókony, V. (2021). Evolutionary and demographic consequences of temperature-induced masculinization under climate warming: The effects of mate choice. BMC Ecology and Evolution, 21, 16. https://doi.org/10.1186/s12862-021-01747-3
Pagliaro, M. D., & Knouft, J. H. (2020). Differential effects of the urban heat island on thermal responses of freshwater fishes from unmanaged and managed systems. Science of the Total Environment, 723, 138084. https://doi.org/10.1016/j.scitotenv.2020.138084.
Perkins-Kirkpatrick, S. E., & Lewis, S. C. (2020). Increasing trends in regional heatwaves. Nature Communications, 11(1), 1–8. https://doi.org/10.1038/s41467-020-16970-7.
Pike, N. (2011). Using false discovery rates for multiple comparisons in ecology and evolution. Methods in Ecology and Evolution, 2(3), 278–282. https://doi.org/10.1111/j.2041-210X.2010.00061.x.
Pottier, P., Burke, S., Drobniak, S. M., Lagisz, M., & Nakagawa, S. (2021). Sexual (in)equality? A meta-analysis of sex differences in thermal acclimation capacity across ectotherms. Functional Ecology, 35(12), 2663–2678. https://doi.org/10.1111/1365-2435.13899.
Preiszner, B., Papp, S., Pipoly, I., Seress, G., Vincze, E., Liker, A., & Bókony, V. (2017). Problem-solving performance and reproductive success of great tits in urban and forest habitats. Animal Cognition, 20(1), 53–63. https://doi.org/10.1007/s10071-016-1008-z.
R Core Team (2022). R: A language and environment for statistical computing. Version 4.2.2. R Foundation for statistical computing, Vienna, Austria. http://www.r-project.org.
Radchuk, V., Reed, T., Teplitsky, C., van de Pol, M., Charmantier, A., Hassall, C., et al. (2019). Adaptive responses of animals to climate change are most likely insufficient. Nature Communications, 10(1), 3109. https://doi.org/10.1038/s41467-019-10924-4.
Riis, N. (1997). Field studies on the ecology of agile frog in Denmark. In A. Krone, K. D. Kühnel, & H. Berger (Eds.), Der Springfrosch (Rana dalmatina). Ökologie und Bestandssituation, Rana Sonderheft 2 (pp. 189–202).
Roeder, K. A., Roeder, D. V., & Bujan, J. (2021). Ant thermal tolerance: a review of methods, hypotheses, and sources of variation. Annals of the Entomological Society of America, 114(4), 459–469. https://doi.org/10.1093/aesa/saab018.
Rohr, J. R., Civitello, D. J., Cohen, J. M., Roznik, E. A., Sinervo, B., & Dell, A. I. (2018). The complex drivers of thermal acclimation and breadth in ectotherms. Ecology Letters, 21(9), 1425–1439. https://doi.org/10.1111/ele.13107.
Rubalcaba, J. G., Gouveia, S. F., & Olalla-Tárraga, M. A. (2019). A mechanistic model to scale up biophysical processes into geographical size gradients in ectotherms. Global Ecology and Biogeography, 28(6), 793–803. https://doi.org/10.1111/geb.12893.
Ruckstuhl, K. E., & Neuhaus, P. (2005). Sexual segregation in vertebrates. Ecology of the two sexes. Cambridge University Press.
Schacht, R., Beissinger, S. R., Wedekind, C., Jennions, M. D., Geffroy, B., Liker, A., et al. (2022). Adult sex ratios: causes of variation and implications for animal and human societies. Communications Biology, 5(1), 1–16. https://doi.org/10.1038/s42003-022-04223-w.
Skelly, D. K., & Freidenburg, L. K. (2000). Effects of beaver on the thermal biology of an amphibian. Ecology Letters, 3(6), 483–486. https://doi.org/10.1046/j.1461-0248.2000.00186.x.
Sykes, B. E., Hutton, P., & McGraw, K. J. (2021). Sex-specific relationships between urbanization, parasitism, and plumage coloration in house finches. Current Zoology, 67(3), 237–244. https://doi.org/10.1093/cz/zoaa060.
Troia, M. J. (2023). Magnitude–duration relationships of physiological sensitivity and environmental exposure improve climate change vulnerability assessments. Ecography, 2023(1), 1–14. https://doi.org/10.1111/ecog.06217.
Turriago, J. L., Tejedo, M., Hoyos, J. M., Camacho, A., & Bernal, M. H. (2023). The time course of acclimation of critical thermal maxima is modulated by the magnitude of temperature change and thermal daily fluctuations. Journal of Thermal Biology, 114(May), 103545. https://doi.org/10.1016/j.jtherbio.2023.103545.
Ujszegi, J., Bertalan, R., Ujhegyi, N., Verebélyi, V., Nemesházi, E., Mikó, Z., et al. (2022). Heat waves experienced during larval life have species-specific consequences on life-history traits and sexual development in anuran amphibians. Science of the Total Environment, 835(March), 155297. https://doi.org/10.1016/j.scitotenv.2022.155297.
Urban, M. C., Richardson, J. L., & Freidenfelds, N. A. (2014). Plasticity and genetic adaptation mediate amphibian and reptile responses to climate change. Evolutionary Applications, 7(1), 88–103. https://doi.org/10.1111/eva.12114.
Vences, M., Hauswaldt, J. S., Steinfartz, S., Rupp, O., Goesmann, A., Künzel, S., et al. (2013). Radically different phylogeographies and patterns of genetic variation in two European brown frogs, genus Rana. Molecular Phylogenetics and Evolution, 68(3), 657–670. https://doi.org/10.1016/j.ympev.2013.04.014.
Zuur, A. F., Ieno, E. N., Walker, J., Saveliev, N., & Smith, G. M. (2009). Mixed effects models and extensions in ecology with R. Springer.
Acknowledgements
We thank Csenge Kalina, Zsanett Mikó, and all other members of the Department of Evolutionary Ecology for their help during the experiment. We are grateful to Bernadett Zsinka (Molecular Ecology Research Group of the University of Veterinary Medicine, Budapest) for help in the DNA work, Julia Halász (Institute of Genetics and Biotechnology, Hungarian University of Agriculture and Life Sciences, Budapest) for help in measuring DNA concentration, and Daniel Teutsch (Quantabio) for the qPCR machine calibration. The study was supported by the National Research, Development and Innovation Office of Hungary (NKFIH K-135016 & K-124375), the New National Excellence Program of the Ministry for Innovation and Technology (ÚNKP-21-5 to V.B. and A.H., ÚNKP-22-5 to A.H., ÚNKP-22-3-1 to E.B., ÚNKP-23-4-II to J.U.), the János Bolyai Scholarship of the Hungarian Academy of Sciences to V.B. and A.H., and the strategic research fund of the University of Veterinary Medicine Budapest (Grant No. SRF-001 to E.B. and V.B.).
Funding
Open access funding provided by HUN-REN Centre for Agricultural Research.
Author information
Authors and Affiliations
Contributions
VB, JU and AH conceived the study; all authors collected the data; VB, EB and AH provided funding; VB analyzed the data and led the writing; all authors contributed with manuscript review and editing.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Bókony, V., Balogh, E., Ujszegi, J. et al. Tadpoles Develop Elevated Heat Tolerance in Urban Heat Islands Regardless of Sex. Evol Biol 51, 209–216 (2024). https://doi.org/10.1007/s11692-024-09626-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11692-024-09626-7