Abstract
Recent studies have found correlations between the shape of snake teeth/fangs and diet. These studies were done at a very broad phylogenetic scale, making it desirable to test if correlations are still detectable at a narrower evolutionary scale, specifically within the family Elapidae. To this end, we studied fang shape in a dense selection of elapids representing most genera worldwide (74%). We used three-dimensional geometric morphometrics to analyse fang diversity and evaluate possible correlations between fang shape, fang size, and diet. We detected weak phylogenetic signal for both shape and absolute fang size, and found that evolutionary allometry contributes a small proportion of the among-species variation. The distribution of elapid fangs in morphospace was found to be surprisingly conservative, with only a few outliers. The only three dietary categories that were found to have a significant effect on fang shape are mammals, lizards, and reptile eggs, with mammals having a significant effect also on absolute but not relative fang size. Our results show that there are disparate patterns in fang-diet relationships at different evolutionary scales. Across all venomous snakes, previous work found that fangs are strongly influenced by diet, but within elapids our study shows these same associations are weaker and often non-significant. This could result from limitations in these types of studies, or could reflect the fact that elapids are a relatively young clade, where recent extensive divergences in diet have yet to be mirrored in fang shape, suggesting a lag between changes in ecology and dental morphology.
Similar content being viewed by others
Introduction
The evolution of fangs and venom delivery systems is considered one of the key innovations underpinning the success and rapid diversification of advanced snakes (Colubroides sensu Zaher et al., 2019), which started to replace larger non-venomous constricting snakes (booids) towards the end of the Oligocene, ~ 25 million years (Myr) ago (Kuch et al., 2006; Westeen et al., 2020). There are currently approximately a thousand species of mildly to highly venomous snakes worldwide, ~ 400 in the family Elapidae, a group of snakes that includes many of the deadliest species, such as cobras, mambas, taipans, and sea snakes (O’Shea, 2005; Valenta, 2010; Figueroa et al., 2016, Uetz et al., 2022).
Enlarged, specialised teeth (venom fangs) are fundamental for the feeding and ecology of snakes that rely on envenomation for prey capture and defence. Elapid fangs are characterised by having an enclosed venom groove, where the two margins of the groove meet in a suture at the front of the tooth. These fangs are typically positioned at the front of the mouth on a (relatively) immobile maxilla, resulting in the "proteroglyphous" arrangement, as opposed to the front hinged "solenoglyphous" fangs of viperids and atractaspidids or the rear "opisthoglyphous" fangs of some colubroides (Greene, 1997; Zaher et al., 2019). Because venom fangs are fundamental for the feeding and ecology of venomous snakes, a strong correlation is expected between fang shape and function, analogous to that observed for mammalian teeth (e.g., Evans & Pineda–Munoz, 2018) or bird beaks (e.g., Cooney et al, 2017).
A recent study that explored correlations between fang shape in snakes and ecological factors (Cleuren et al., 2021) concluded that fang shape and size are very good predictors of diet, based on a broad sample of venomous snakes representing four major families (2 species of Colubridae, 51 Elapidae, 4 Homalopsidae, 24 Viperidae). Slender elongate fangs with sharp tips are typical of snakes that feed on soft-skinned prey (mammals and birds), while more robust and blunter fangs are typical of snakes that feed on scaly prey (fish, reptiles or arthropods). A similar conclusion was reached by Segall et al. (2023), who evaluated dentary tooth shapes across venomous and non-venomous snakes. These authors also concluded that in snakes tooth shape is strongly driven by ecological factors, and recognised two main tooth shapes, short and robust vs long and slender. Long teeth are typical of snakes that require a good grip on soft-bodied prey or feed underwater, while short teeth would be associated with hard and/or long prey. While an important study of general tooth shape in snakes, Segall et al.’s (2023) study focused on dentary teeth and included only 8 elapids out of the 63 species sampled. In this study we wanted to focus on fang shape within elapids, because of their ecological diversity and also because of their close phylogenetic relatedness and recent divergences (i.e., within the last 30 My; Zaher et al., 2019), which makes potential ecological signals much easier to identify as they are less conflated by phylogenetic history. Therefore, the main goal of this study was to see whether the above-mentioned general patterns are also manifest in elapid fangs when considered separately from other venomous snakes.
Materials and Methods
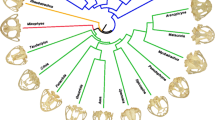
We sampled 70 elapid species, representative of all major global clades and 42 out of 57 genera (i.e., 74% of the genera following the classification of Uetz et al., 2022) (supplementary material, File S1). All specimens sampled were from adult individuals, based both on size and morphology (one individual per species). Sex was not factored in, as sexually dimorphic dietary divergence is rarely documented in elapids, e.g., red-bellied blacksnakes and sea kraits (Shine 1991; Shetty and Shine, 2002; but even in these cases the broad diet categories used here would be same for both sexes). Some of the most speciose genera (e.g., Micrurus, Hydrophis, Naja) were sampled for multiple species to account for possible intrageneric variation, but their number was still limited to no more than six in order to avoid statistical bias (i.e., even accounting for phylogenetic history, sampling several sea snakes that all feed on fish would potentially bias statistical testing for dietary effects on fang shape: Uyeda et al., 2018).
High resolution computed tomography (microCT) data for these species were obtained from a variety of sources including new original scans. Part of the microCT data was sourced from the digital collections of the South Australian Museum in Adelaide (SAMA), the digital collections of the University of Michigan Museum of Zoology in Ann Arbor (UMMZ), Morphosource.org, and the supplementary data from Chen et al. (2021). The remaining taxa were microCT scanned for the purpose of this study and all scans are available on Morphosource.org (project ID: 00000C744, “Elapid Snakes"). These specimens were all CT scanned using a Bruker Skyscan 1276 high-resolution system at Adelaide Microscopy (Adelaide Health and Medical Sciences Building, Adelaide, Australia). The acquired images were then processed in NRecon v. 1.7.3.1 (Bruker microCT), to obtain 2D cross sections (slices) as .bmp files. Avizo v.2020.1 (Thermo Fisher Scientific) was used to produce digital surface models (.ply) of the right maxillary bones. When the fang on the right maxillary bone was damaged or missing, the left maxilla was isolated instead and then mirrored in Checkpoint v. 2020.10.13.0859 (Stratovan Corporation).
To compare fang shape in our taxonomic sample, we selected a landmarking scheme that is effective at capturing curvature and thickness of the fangs, as well as the size and position of the venom orifices (Fig. 1). This landmarking scheme included 9 fixed landmarks and 18 sliding semilandmarks. The surface models of the fangs were landmarked in 3D using Checkpoint v. 2020.10.13.0859. The landmark coordinates were saved in NTSYS format and then imported into R Statistical Environment v.1.4.1106 (R Core Team, 2021), using the ‘readland.nts’ function in the R package geomorph v.4.0.3 (Adams et al., 2021).
Landmark configurations were superimposed using Generalised Procrustes Analysis (GPA) using the ‘gpagen’ function in the R package geomorph. Semilandmarks were first standardised using ‘digit.curves’ in geomorph (see supplementary data), and then permitted to slide during Procrustes superimposition by minimizing bending energy. Fang size was approximated by the centroid size of the landmark configuration, i.e., by the square root of the sum of the squared distances of all landmarks from their centroid (Kendall, 1984; Zelditch et al., 2012), calculated during superimposition.
In order to quantify measurement error in the landmark procedure, a Procrustes ANOVA was performed on 5 randomly selected specimens, each landmarked 5 times to verify that variance between replicates was negligible compared to variance between specimens. The repeatability value (R), or intraclass correlation coefficient of Fisher (1954), was then calculated. This is the ratio of the among-individual variance and the sum of the among-individual variance and measurement error component (see also Zelditch et al., 2012). The specimens selected were: Drysdalia mastersi (SAMA R50230), Hydrophis elegans (WAM R174264), Naja naja (UMMZ H61298), Ophiophagus hannah (UMMZ H239131), and Suta suta SAMA R20543 (for full list of institutional abbreviations see supplementary material, File S1).
Principal component analyses (PCA) were used to visualize the distribution of fang shapes in morphospace (Zelditch et al., 2012). The PCA were performed using the function ‘gm.prcomp’ from the package geomorph and plotted using the ‘plot’ function. Warped surfaces derived from the fang closest to the average shape were used to visualize shape transformations along the minima and maxima directions of the principal components (PCs). These warped surfaces were created using the ‘plotRefToTarget’ function in the geomorph package.
To account for phylogenetic relatedness, we used the the ultrametric tree with branch lengths from Maritz et al. (2021); these branch lengths are putative molecular divergence dates in millions of years (Tonini et al., 2016). The phylogeny was pruned of unsampled taxa using Mesquite v. 3.51 (Maddison & Maddison, 2018) and then imported into R using the function ‘read.tree’ from the package ape (Paradis et al., 2004). The presence of phylogenetic signal was tested using the function ‘physignal’ from the package geomorph, which calculates Kmult, a multivariate version of Blomberg et al. (2003) K statistic (Adams, 2014a). Significance was assessed through 10,000 permutations. Phylogenetic signal was tested for shape, absolute fang size (fang centroid size), and relative fang size (i.e., fang centroid size/mandible length).
Presence of evolutionary allometry in the data was tested using a multivariate regression of shape vs size (as logged centroid size) while keeping into account also phylogenetic information derived from the pruned tree in Maritz et al. (2021) (see above). The phylogenetic regression was done using the function ‘procD.pgls’ from the package geomorph, which evaluates a phylogenetic generalised least squares (PGLS) model suitable for multivariate data (Adams, 2014b). The regression was plotted using the regression score approach (Drake & Klingenberg, 2008) versus logged centroid size.
The effect of diet on fang shape was tested simultaneously for all dietary categories, using a phylogenetically informed multifactor ANOVA (sum of squares Type III) and 10,000 permutations for significance testing implemented with the function ‘procD.pgls’. Diet data were taken from Maritz et al. (2021), but where terrestrial and marine invertebrates were merged into the same category, because only three snakes (sea snakes) in our sample feed on marine invertebrates. The dietary category “fish eggs” was not evaluated because in our sample only two closely related sea snakes are fish-egg eaters (two species of Aipysurus). Thus, the dietary categories evaluated were: birds, mammals, lizards, snakes, amphibians, fish, invertebrates, reptile eggs and bird eggs. The effect of diet on fang size was also tested with the function ‘procD.pgls’, again using a phylogenetically informed multifactor ANOVA (sum of squares Type III) and 10,000 permutations for significance testing.
In order to control for snake head size and discriminate between fangs that were large in absolute vs relative size, we also took measurements of the jaw length (mandible) for each snake specimen in our sample. Jaw length was measured in Avizo v.2020.1 (Thermo Fisher Scientific) whenever the microCT scan dataset included the whole skull, and with a digital caliper on the actual specimens when this was not the case (i.e., when only the fangs had been microCT scanned). In all cases, jaw length was measured between the anterior end of the dentary and the posterior end of the retroarticular process of the compound bone, and the average between left and right sides was calculated.
Snake species were classified based on the number of their dietary categories (ranging 1 to 9) to differentiate between specialists and generalists. Then phylogenetic ANOVAs were run to test for an effect of the number of dietary categories on fang shape and size (the tree used was again the pruned version from Maritz et al. [2021]), to evaluate whether generalist snakes differ or tend to have a similar fang shape or size.
We measured morphological disparity, measured as the Procrustes variance, overall and for dietary groups, using residuals of a linear model fit. A disparity measurement for the whole sample was obtained using the ‘morphol.disparity’ function in the R package geomorph and the following linear model: shape ~ 1, where ‘shape’ is the array containing the landmark coordinates after general Procrustes alignment. Because overall disparity alone may not be very meaningful, we also compared disparities between groups (shape variance). The groups were based on diet, but each dietary category was tested separately (e.g., fish-eaters vs non-fish-eaters). This was done with the caveat in mind that diets exhibit considerable overlap, i.e., each species can span multiple diet categories.
To evaluate the tempo and mode of evolution in our sample we used the dated tree from Lee et al. (2016). However, the latter does not include all of the taxa in our sample, so some had to be replaced by close relatives and five had to be excluded (supplementary data, File S1). The tree was edited in Mesquite v. 3.51 (Maddison and Maddison, 2018) and then imported into R using the function ‘read.tree’ from the R package ape. Rates of morphological change for each species were calculated as the magnitude of shape change estimated from Procrustes distances along each internal and terminal branch, using a maximum likelihood ancestral estimation of each node calculated within geomorph’s ‘gm.prcomp’, which was used to plot a phylomorphospace to visualise the evolutionary history (mode) of fang shape in morphospace. The branch lengths in Procrustes distance were then divided by the square-root of the original branch length in unit time, to relate phenotypic divergence to branch duration in a maximum likelihood framework (Felsenstein, 1973). To visualise the rates of morphological change along each branch we used ‘plotBranchbyTrait’ function in phytools R package v. 1.5-1 (Revell, 2012).
To evaluate whether accelerated rates of fang shape change were associated with accelerated rates of dietary shifts, we mapped rates of diet change onto the same dated phylogeny (Lee et al. 2016). The nine dietary items were scored as binary characters (absence or presence), and the overall rate of change across all nine characters on each branch estimated using the Mk model (Lewis, 2001) and an uncorrelated lognormal relaxed clock (Drummond et al., 2006) using BEAST (Suchard et al., 2018). Four runs (2 million steps, burnin of 20%) were performed and convergence confirmed (ESS > > 200) using Tracer (Rambaut et al., 2018).
Results
The test of measurement error using a Procrustes ANOVA on 5 randomly selected specimens landmarked 5 times each returned a strongly significant p-value (p = 0.0001), meaning that the variance within groups (caused by landmarking error) is negligible compared to the variance between groups (specimens). The repeatability value (Zelditch et al., 2012) was R2 = 0.93. This confirms the validity and efficacy of our landmarking scheme in capturing actual shape variation among individuals.
An overview of fang shape distribution in the morphospace described by the first two principal components (PC1 and PC2) is shown in Fig. 2. Most taxa tend to be clustered around the mean shape (between − 0.1 and + 0.1 on both axes), with only a few outliers (labelled in Fig. 2). The first two components together explain about 47% of the total shape variance, with PC3 explaining another 13%.
PC1 describes both robustness and curvature of the fangs (slenderer and more recurved at higher values), and also the relative position of the proximal venom orifice, which is closer to the base of the crown for higher values of PC1. PC2 describes robustness (broader vs narrower base) and the orientation of the attachment area, with the medial wall higher for negative PC2 and lower for positive PC2. This corresponds to a change in inclination of the fangs, which shift from pointing slightly inward (e.g., Simoselaps bimaculatus) to pointing slightly outward (e.g., Laticauda colubrina) (supplementary material, File S2 Fig. S1). PC3 describes mostly curvature of the fangs, with more recurved fangs at higher values, but also the shape of the proximal venom orifice, which is rounder for higher values and more elongate dorsoventrally at lower values (Fig. 2).
Principal Component (PC) analysis plots showing the three main axes of fang shape variation (PC1 vs PC2 and PC1 vs PC3). Point size is proportional to relative fang size (i.e., fang centroid size/mandible length), illustrating that no one axis is driven by allometric variation. Warped 3D surfaces of the fangs are shown beside the axes to describe shape transformations towards the extremes of each axis (in right lateral and anterior views). Outlying taxa are labelled.
There is a significant phylogenetic signal in the fang shape data (Kmult= 0.49, p = 0.0004), but this is probably caused by a few outlying clades that stand out from the cluster, as shown by the phylomorphospace (supplementary material, File S2 Fig. S2). The low Kmult value (< 1) indicates that phylogenetic signal is less than that expected under a Brownian motion model of evolution, though significant. A similar result was found for absolute fang size (Kmult= 0.68, p = 0.0082). On the other hand, phylogenetic signal in relative fang size was found to be higher and consistent with what should be expected under a Brownian motion model of evolution (Kmult = 1.03, p = 0.0003).
A phylogenetically informed regression of shape on absolute size returned a non-significant result (R2 = 0.01073, p = 0.5277), meaning that there is no statistical support for evolutionary allometry in the shape data using a phylogenetic regression model (supplementary material, File S2 Fig. S3). However, a non-phylogenetic regression found there to be significant evolutionary allometry (R2 = 0.10177, p < 0.001), which is consistent with size and phylogenetic relatedness covarying (i.e., phylogenetic signal in fang size).
The test of an effect of diet on fang shape returned three significant results for the dietary categories of mammals (R2 = 0.03455, p = 0.0038), lizards (R2 = 0.02687, p = 0.0309), and reptile eggs (R2 = 0.02698, p = 0.0420). However, even when having a significant effect, diet appears to explain very little of the overall shape variance (i.e., less than 5%). Based on fang distribution in morphospace, mammal eaters tend to have fangs that are thin and slender, and with various degrees of curvature (i.e., mostly absent from negative quadrants of PC1 and PC2; cf. Figures 2 and 3). Snake eaters tend to have fangs that appear distributed randomly in the first three principal components, but do not occur in areas with high values of PC2 (i.e., above 0.08), which implies that they do not take a slender, weakly recurved shape with a point directed laterally. Reptile-egg eaters tend to have fangs that are mostly distributed around mean values of PC1, but can spread out along both PC2 and PC3. This mostly corresponds to fangs that are of average curvature and slenderness (cf. Figures 2 and 3).
Regarding the effect of diet on fang size (absolute size), there is a significant effect only in snakes that feed on mammals (R2 = 0.10241, p = 0.0072). Most mammal-eaters have medium to large fangs, with rare exceptions such as Suta suta and Elapsoidea semiannulata (Fig. 3). Fish-eaters have fangs that are almost significantly larger than average (R2 = 0.05012, p = 0.0512), but with enough exceptions to make the effect non-significant (see supplementary material, File S2 Fig. S4 for the distribution of fish-eaters in morphospace and their spread of fang sizes).
It should be noted that snakes such as Suta suta and Elapsoidea semiannulata are small-bodied snakes, hence they have very small fangs. To keep this into account, we divided fang size (centroid size) by mandible length, in order to calculate a relative fang size. The variation in relative size (Fig. 2) is considerably less evident in the PCA compared to variation in absolute size (Fig. 3). When we tested for an effect of prey type on relative fang size (i.e., corrected for mandible length) we obtained no significant associations.
Statistically significant effects of diet on fang shape: distribution in morphospace (PC1 vs PC2 and PC1 vs PC3) of snakes with a predominant diet of mammals, lizards, and/or reptile eggs. Point size is proportional to absolute fang size (centroid size), contrasting relative size in Fig. 2. A diet of mammals was found to also have a significant effect on fang size (R2 = 0.10241, p = 0.0072). Significance was tested using a phylogenetic multifactor ANOVA (see text for details).
The test for an effect of generalist diets vs specialized diets found that the number of dietary categories did not have a significant effect on either fang shape (R2 = 0.01826, p = 0.2895) or fang size (R2 = 0.02873, p = 0.1638). Regardless of generalist or specialist dietary habits, fang shape can occupy highly overlapping regions of morphospace (supplementary material, File S2 Fig. S5).
Overall disparity for the sample, i.e., the Procrustes variance from the mean, was found to be 0.0138, a relatively low value consistent with the observation that most points in morphospace are closely clustered around the mean with only a few outliers (Fig. 2). Regarding disparity for the individual dietary groups, snakes that feed on invertebrates appear to have the lowest disparity, which might suggest some shape constraint for them to be able to feed on that type of prey, while snakes that feed on bird eggs tend to have the highest disparity. The low disparity of invertebrate-eaters is not explained by close phylogenetic relationships, because these snakes are spread across the elapid phylogeny (supplementary material, File S2 Fig. S6). In any case, all these differences in disparity were found to be minimal (disparities were all in the range 0.0110–0.0160) (supplementary material, File S2 Fig. S7).
The magnitude of variation along the phylomorphospace branches scaled by the time-calibrated branch lengths was plotted onto the pruned dated phylogeny of Lee et al. (2016) and the results are shown in Fig. 4. Many lineages evolved at broadly similar rates (purple branches in Fig. 4), but some evolved notably faster than average (red branches in Fig. 4). These fast-evolving lineages include the two coral snakes Micrurus obscurus and M. tschudii, the two spitting cobras Naja siamensis and N. sumatrana, the sea krait Laticauda colubrina, the invertebrate specialist Toxicocalamus loriae, Brachyurophis semifasciatus, Oxyuranus scutellatus, the sea snakes in the genus Hydrophis (especially H. elegans and H. curtus), the two sea snakes in the genus Aipysurus, and the semiaquatic Hydrelaps darwiniensis.
In general, there is no apparent correspondence between rates of change in diet and branches showing greater magnitude of shape variation (supplementary material, File S2 Fig. S8).
Magnitude of shape variation along the phylomorphospace branches scaled by the time-calibrated branch lengths. Scale bar on the left shows colours associated with rates of Procrustes distance (PD) per million years (Ma) (warmer colours indicate faster rates of shape change). Dated phylogeny modified from Lee et al. (2016).
Discussion
Our study complements previous studies (e.g., Cleuren et al., 2021; Segall et al., 2023) that found significant diet-tooth shape relationships across all venomous snakes, but finds that such patterns are weaker (and often insignificant) when more recent evolutionary divergences are considered separately, i.e., within Elapidae alone. In fact, unlike venomous snakes as a whole (Cleuren et al., 2021), fang shape in elapid snakes appears to be fairly conservative, and most species sampled were clustered in morphospace closely around the mean (Fig. 2). This could be the result of phylogenetic history, since elapids diverged from the most recent common ancestor with their sister group within Colubroides only about 30 Mya (Zaher et al., 2019). This interpretation seems supported by our finding of a significant phylogenetic signal in the data. However, if we look at the phylomorphospace (supplementary material, File S2 Fig. S2), it is clear that there is extensive overlap across clades, with two outliers in the same direction from a single clade which may cause the phylogenetic signal to be retrieved as overly significant (p = 0.0005) (Uyeda et al., 2018). In fact, Blomberg’s Kmult value was found to be 0.48, meaning that phylogenetic signal in our data is substantially less than that expected under a Brownian motion (BM) model of evolution (Adams, 2014a). This suggests that a BM model may not be adequate for these data and the phylogenetic comparative methods used herein for highly multivariate data that assume a BM model are not strictly appropriate. However, another possibility is that evolution has been (approximately) Brownian, but our rate analyses are unable to reconstruct the full extent of shape changes on deep nodes resulting in apparent slower rates in basal branches (Brocklehurst et al. 2021).
Evolutionary allometry accounted for approximately 10% of the variation observed among species, which is consistent with the findings of fang shape by Cleuren et al. (2021) across the fangs of venomous snakes in general and fang size by Holding et al. (2022) for viperids. However, only a non-phylogenetic regression model of shape and size yielded a significant result, which indicates that one or more variables are strongly correlated with phylogeny. In fact, we found significant phylogenetic signal in both fang shape and fang size (both absolute and relative). Strong phylogenetic signal in fang size can impede detection of evolutionary allometry with PGLS approaches because phylogenetic structure along the independent variable of a regression model will render any allometric component of shape not statistically significant (e.g., Uyeda et al., 2018; Rohlf, 2006). The biological outcome is arguably better described in these situations by a non-phylogenetic model.
Regarding the relationship between diet and fang shape, we found that only diets inclusive of mammals, lizards or reptile eggs have a significant effect, again in contrast to the stronger patterns observed across all venomous snakes by Cleuren et al. (2021), where most tested prey categories were found to have a significant effect on fang shape. Moreover, even if significant, the amount of shape variance explained by diet was always minimal (< 5%). In their study of colubriform snakes, Westeen et al. (2020) did not analyse the effect of diet on fang shape directly, but they also found that there is only a weak signal between diet and dental phenotype, expressed as maxillary length and tooth counts.
Similar to the results in Cleuren et al. (2021) across all venomous snakes or Holding et al. (2022) for viperids, we found that in elapids, when considered separately from other venomous clades, diet can indeed have a significant effect on fang size, but only when it includes mammals and possibly fish (the effect of fish on fang size was almost significant, p = 0.05). However, these relationships were no longer significant after correcting for snake size (i.e., after dividing fang size by mandible length). To subdue their prey, snakes that feed on mammals tend to be large overall, and as a consequence their fangs are larger than average in absolute size, but the relative size of their fangs is not significantly larger than that of snakes that feed on other types of prey. This is unlike what was observed by Holding et al. (2022) for vipers, which show distinct allometry in fang size relative to head size. These results highlight the importance of the distinction between relative and absolute size, and why they should be considered both separately and alongside each other (Klingenberg, 2016).
Cleuren et al. (2021) had also found that generalist snakes, i.e., snakes that feed on a wide variety of prey items, tend to resemble in fang shape, showing an intermediate level of robustness. The results from our elapid dataset could not confirm this observation within this clade, and snakes were mostly clustered around the mean regardless of their being generalists or specialists. The phylogenetic ANOVA also returned a non-significant effect of the number of prey categories on both fang shape and size.
Consistent with the limited spread across morphospace observed for elapid fangs, we found that morphological disparity in our dataset is very limited. Diet does not appear to strongly affect disparity, as all values of disparity are very similar and range between 0.0110 and 0.0160. This again confirms the relatively constrained nature of fang shape in elapids.
We calculated the magnitude of variation along the phylomorphospace branches scaled by the time-calibrated branch length for all the lineages in our sample. The results are shown is Fig. 4. Most rates of change appear relatively similar, but with a few notable exceptions. While it is unclear why fang shape in these lineages evolved faster, it is possible that morphological adjustments for spitting are causing the faster rates in the two Naja species. However, N. mossambica and N. nigricollis are also spitting cobras, but neither of these have unusually fast rates. In fact, in terms of general fang shape all the Naja species tend to be quite similar, and with the only exception of N. nivea all fall relatively close together in the morphospace defined by the first three PC axes, occupying the positive quadrants for PC1, PC2 and PC3.
Overall, the fangs of elapid snakes are characterized by a general uniformity when compared to those of venomous snakes as a whole. Most of the results found across all venomous snakes (Cleuren et al., 2021) were not apparent in our study centred around elapid snakes alone. This result parallels that in mammals, where diet can be inferred from dental morphology in mammals as a whole (e.g., Pineda-Munoz et al., 2017; Evans & Pineda-Munoz, 2018), but tooth shape is a poor predictor of diet in carnivorans alone (Hopkins et al., 2022).
These contrasting patterns at different evolutionary scales are notable, and there are (at least) three broad explanations, which are not mutually exclusive. The first possible explanation relates to the time it might take for form-function evolutionary relationships to manifest. Elapids are a young clade, and recent ecological and dietary divergence has perhaps not yet been strongly reflected in fang shape. In other words, there could be some degree of lag between diet evolution and tooth shape evolution. In elapids, extensive ecological and dietary divergence has already occurred in their relatively short history, but fang shape remains more uniform and changes in dentition may not have ‘caught up’ yet, with only a few exceptions. A lag in the evolution of morphology was also hypothesised to be the cause of the mismatch between tooth shape and diet in carnivorans by Hopkins et al. (2022). According to Hopkins et al. (2022) dental morphology is constrained by genetic factors early in development, and thus will adapt slowly to dietary changes occurring between different environments, between individuals within a population, or over the lifetime of a single individual (but see Cleuren et al. [2022] regarding ontogenetic changes in fang shape observed in the elapid snake Pseudonaja affinis).
A second explanation for this poor correlation between diet and fang shape or size could be caused by weak phylogenetic signal in the elapid fang data. In fact, a stronger phylogenetic signal may be driving some of the significance in venomous snakes overall, possibly influencing the results. For example, most vipers feed on mammals, with several species specializing on them (Holding et al., 2022), and have very particularly shaped fangs, i.e., very long and slender (Cleuren et al., 2021). While Cleuren et al. (2021) took phylogenetic information into account, the presence of a large clade of snakes (24 of the 81 species in their sample), most members of which have mammals in their diet and also possess a very distinctive fang anatomy, may have contributed to clearer associations (Uyeda et al., 2018).
A third possible explanation relates to violation of one or more assumptions that underlie studies attempting to relate form to function (Feilich & López-Fernández (2019). Three of these assumptions might be particularly relevant in studies of snake fangs: 1) that morphology is generally adaptive; 2) that the morphology of interest has a single consistent mapping to function across study organisms; and 3) that if individuals or species are being assigned to categorical bins describing some aspect of their ecology, these bins are accurate in their reflection of life history.
Regarding the first assumption, even if morphology is adaptive, the specific traits studied may not reflect the aspects of ecology that we are considering (e.g., diet). This point is quite relevant for fang shape, because we found that even when there was a significant effect of diet on fang shape, this effect was very small (< 5%), with other unknown factors, e.g., bite biomechanics, different striking behaviour strategies (Broeckhoven & du Plessis, 2017), or enamel thickness (Dumont et al., 2023), potentially contributing to the rest of the variation. If morphology is indeed adaptive, then we should also expect to see accelerated morphological divergence (lack of stasis) accompanying ecological diversification (e.g., Mahler et al., 2010; Lopez–Fernandez et al., 2013; Eliason et al., 2021). Our quantification of magnitude of variation in elapid snake fangs along the phylomorphospace branches showed that accelerated divergence of fang shape was not obviously associated with rates of change in diet: several branches that show a rapid shift in diet do not show a corresponding faster rate of fang shape evolution (supplementary material, File S2, Fig. S8).
The second assumption concerns the belief that the relationship between form and function can be extended to the whole range of organisms being studied, but this may not be the case. As noted by Feilich & López-Fernández (2019:364) “there are extreme cases where, even when taxa are related, their functional construction may diverge to such a degree that different clades become simply non-comparable”. This applies very well to the condition in viperids and elapids, where fangs have a very different construction, the former being hinged and rotatable in a vertical plane, and the latter being fixed at the front of the mouth. The biomechanical stresses these two types of fangs have to cope with are unavoidably quite different and may affect shape much more than other factors such as diet. It is also possible that the fangs of viperids are less constrained in terms of size, because they can be folded into the mouth when not in use.
Finally, the third assumption revolves around the fact that ecological bins, such as dietary categories, are not always accurate. This could be the case with snake diets, where information is often very limited and there is a large amount of continuity and overlap (i.e., diet components are not mutually exclusive), so that for example “mammal-eaters” will rarely feed only on mammals, but also include several other prey items in their diet, confounding inferences about a specific correlation between fang shape and feeding on mammals. Including more detailed dietary information (e.g., percentage of various diet categories) could mitigate this issue. However, this information is not currently available for many snakes and when available is often limited to only a few sampled specimens per species, which may not be representative of the diet of the species as a whole (e.g., prey preference/availability can vary by locality, sex, or age/size of the snakes) (e.g., Daltry et al., 1998; Luiselli, 2006; Manjarrez et al., 2017). In summary, this is a limitation that is hard, if not impossible, to circumvent, unless one limits the study to include only taxa with extremely specialised diets, but this would greatly reduce sample size and, consequently, affect meaningful statistical inferences.
Violation of one or more of these assumptions may provide an explanation of the divergent results produced by our study as compared to the general studies of snake tooth shape (Cleuren et al., 2021; Segall et al., 2023) or the study of fang length in vipers (Holding et al., 2022).
It should be noted that the explanations we discussed are not mutually exclusive. However, regardless of the cause at the root of this discrepancy, our study places some caveats on suggestions that fang shape can be used to infer diet in living and fossil snakes (Cleuren et al., 2021), and that these inferences can be used, for example, to make informed decisions on the selection of new habitats for relocation of a snake population, or on how to protect endangered prey species from putative snake predators. At the appropriate scale (e.g., all venomous snakes: Cleuren et al., 2021), fang-diet inferences might be justified, but at smaller scales (i.e., within families) they become problematic, in a way analogous to what has been found for carnivorans in the broader context of mammals dental morphology (Hopkins et al., 2022). Palaeoecological inferences can be plagued by the same uncertainties, at least at particular scales (e.g., elapids within snakes or carnivorans within mammals). As discussed above, this uncertainty in elapids is probably caused by a series of factors, such as the lag between ecological diversification and fang evolution, the relative conservativeness of fang shape, particular biomechanical properties of elapid fangs (i.e., fixed front fangs, as opposed to hinged front fangs or fixed rear fangs), different striking behaviours (bite and hold vs strike and release), and the complex overlapping diets that are challenging to quantify.
Data Availability
All specimen landmark data and analysis code are included in the submission as supplementary data, available at https://doi.org/10.25909/23683422. The CT scan data acquired for this study is available on Morphosorce.org (project ID: 00000C74, “Elapid Snakes”).
References
Adams, D. C. (2014a). A generalized K statistic for estimating phylogenetic signal from shape and other high-dimensional multivariate data. Systematic Biology, 63, 685–697. https://doi.org/10.1093/sysbio/syu030.
Adams, D. C. (2014b). A method for assessing phylogenetic least squares models for shape and other high-dimensional multivariate data. Evolution, 68, 2675–2688. https://doi.org/10.1111/evo.12463.
Adams, D. C., Collyer, M. L., Kaliontzopoulou, A., & Baken, E. K. (2021). Geomorph: Software for geometric morphometric analyses. R package version 4.0. https://cran.r-project.org/package=geomorph.
Blomberg, S. P., Garland, T. Jr., & Ives, A. R. (2003). Testing for phylogenetic signal in comparative data: Behavioral traits are more labile. Evolution, 57, 717–745. https://doi.org/10.1111/j.0014-3820.2003.tb00285.x
Broeckhoven, C., & du Plessis, A. (2017). Has snake fang evolution lost its bite? New insights from a structural mechanics viewpoint. Biology Letters, 13, 20170293. https://doi.org/10.1098/rsbl.2017.0293.
Brocklehurst, N., Panciroli, E., Benevento, G. L., & Benson, R. B. (2021). Mammaliaform extinctions as a driver of the morphological radiation of Cenozoic mammals. Current Biology, 31, 2955–2963. https://doi.org/10.1016/j.cub.2021.04.044.
Chen, Z. N., Shi, S. C., Vogel, G., Ding, L., & Shi, J. S. (2021). Multiple lines of evidence reveal a new species of Krait (Squamata, Elapidae, Bungarus) from Southwestern China and Northern Myanmar. ZooKeys, 1025, 35.
Cleuren, S. G., Hocking, D. P., & Evans, A. R. (2021). Fang evolution in venomous snakes: adaptation of 3D tooth shape to the biomechanical properties of their prey. Evolution, 75, 1377–1394. https://doi.org/10.1111/evo.14239.
Cleuren, S. G., Patterson, M. B., Hocking, D. P., Warburton, N. M., & Evans, A. R. (2022). Fang shape varies with ontogeny and sex in the venomous elapid snake Pseudonaja affinis. Journal of Morphology, 283, 287–295. https://doi.org/10.1002/jmor.21442.
Cooney, C. R., Bright, J. A., Capp, E. J., Chira, A. M., Hughes, E. C., Moody, C. J., Nouri, L. O., Varley, Z. K., & Thomas, G. H. (2017). Mega-evolutionary dynamics of the adaptive radiation of birds. Nature, 542, 344–347. https://doi.org/10.1038/nature21074.
Daltry, J. C., Wüster, W., & Thorpe, R. S. (1998). Intraspecific variation in the feeding ecology of the crotaline snake Calloselasma rhodostoma in Southeast Asia. Journal of Herpetology, 32, 198–205. https://doi.org/10.2307/1565297.
Drake, A. G., & Klingenberg, C. P. (2008). The pace of morphological change: historical transformation of skull shape in St Bernard dogs. Proceedings of the Royal Society B: Biological Sciences, 275, 71–76.
Drummond, A., Ho, S. Y. W., Phillips, M. J., & Rambaut, A. (2006). Relaxed phylogenetics and dating with confidence. PloS Biology, 4, e88. https://doi.org/10.1371/journal.pbio.0040088.
Dumont, M., Milgram, J., Herrel, A., Shahar, R., Shacham, B., Houssin, C., Delapré, A., Cornette, R., & Segall, M. (2023). Show me your teeth and I will tell you what you eat: differences in tooth enamel in snakes with different diets. Integrative and Comparative Biology. https://doi.org/10.1093/icb/icad028
Eliason, C. M., McCullough, J. M., Andersen, M. J., & Hackett, S. J. (2021). Accelerated brain shape evolution is associated with rapid diversification in an avian radiation. The American Naturalist, 197, 576–591. https://doi.org/10.1086/713664.
Evans, A. R., & Pineda-Munoz, S. (2018). Inferring mammal dietary ecology from dental morphology. In D. A. Croft, D. F. Su, & S. W. Simpson (Eds.), Methods in Paleoecology: Reconstructing Cenozoic terrestrial environments and ecological communities (pp. 37–51). US: Springer.
Feilich, K. L., & López-Fernández, H. (2019). When does form reflect function? Acknowledging and supporting ecomorphological assumptions. Integrative and Comparative Biology, 59, 358–370. https://doi.org/10.1093/icb/icz070.
Felsenstein, J. (1973). Maximum likelihood estimation of evolutionary trees from continuous characters. American Journal of Human Genetics, 25, 471–492.
Figueroa, A., McKelvy, A. D., Grismer, L. L., Bell, C. D., & Lailvaux, S. P. (2016). A species-level phylogeny of extant snakes with description of a new colubrid subfamily and genus. PloS One, 11, e0161070. https://doi.org/10.1371/journal.pone.0161070.
Greene, H. W. (1997). Snakes: The Evolution of Mystery in Nature. University of California Press.
Holding, M. L., Trevine, V. C., Zinenko, O., Strickland, J. L., Rautsaw, R. M., Mason, A. J., Hogan, M. P., Parkinson, C. L., Grazziotin, F. G., Santana, S. E., & Davis, M. A. (2022). Evolutionary allometry and ecological correlates of fang length evolution in vipers. Proceedings of the Royal Society B, 289, 20221132. https://doi.org/10.1098/rspb.2022.1132.
Hopkins, S. S., Price, S. A., & Chiono, A. J. (2022). Influence of phylogeny on the estimation of diet from dental morphology in the Carnivora. Paleobiology, 48, 324–339. https://doi.org/10.1017/pab.2021.37.
Kendall, D. G. (1984). Shape manifolds, procrustean metrics, and complex projective spaces. Bulletin of the London Mathematical Society, 16, 81–121. https://doi.org/10.1112/blms/16.2.81.
Klingenberg, C. P. (2016). Size, shape, and form: concepts of allometry in geometric morphometrics. Development Genes and Evolution, 226, 113–137. https://doi.org/10.1007/s00427-016-0539-2.
Kuch, U., Müller, J., Mödden, C., & Mebs, D. (2006). Snake fangs from the Lower Miocene of Germany: evolutionary stability of perfect weapons. Naturwissenschaften, 93, 84–87. https://doi.org/10.1007/s00114-005-0065-y.
Lee, M. S. Y., Sanders, K. L., King, B., & Palci, A. (2016). Diversification rates and phenotypic evolution in venomous snakes (Elapidae). Royal Society Open Science, 3, 150277. https://doi.org/10.1098/rsos.150277.
Lewis, P. O. (2001). A likelihood approach to estimating phylogeny from discrete morphological character data. Systematic Biology, 50, 913–925. https://doi.org/10.1080/106351501753462876.
Lopez-Fernandez, H., Arbour, J. H., Winemiller, K. O., & Honeycutt, R. L. (2013). Testing for ancient adaptive radiations in neotropical cichlid fishes. Evolution, 67, 1321–1337.
Luiselli, L. (2006). Broad geographic, taxonomic and ecological patterns of interpopulation variation in the dietary habits of snakes. Web Ecology, 6, 2–16. https://doi.org/10.5194/we-6-2-2006.
Maddison, W. P., & Maddison, D. R. (2018). Mesquite: a modular system for evolutionary analysis, Version 3.51. Available at: http://www.mesquiteproject.org.
Mahler, D. L., Revell, L. J., Glor, R. E., & Losos, J. B. (2010). Ecological opportunity and the rate of morphological evolution in the diversification of Greater Antillean anoles. Evolution, 64, 2731–2745. https://doi.org/10.1111/j.1558-5646.2010.01026.x.
Manjarrez, J., Pacheco-Tinoco, M., & Venegas-Barrera, C. S. (2017). Intraspecific variation in the diet of the Mexican garter snake Thamnophis eques. PeerJ, 5, e4036. https://doi.org/10.7717/peerj.4036.
Maritz, B., Barends, J. M., Mohamed, R., Maritz, R. A., & Alexander, G. J. (2021). Repeated dietary shifts in elapid snakes (Squamata: Elapidae) revealed by ancestral state reconstruction. Biological Journal of the Linnean Society, 134, 975–986. https://doi.org/10.1093/biolinnean/blab115.
O’Shea, M. (2005). Venomous Snakes of the World. Princeton University Press.
Paradis, E., Claude, J., & Strimmer, K. (2004). APE: analyses of phylogenetics and evolution in R language. Bioinformatics, 20, 289–290. https://doi.org/10.1093/bioinformatics/btg412.
Pineda-Munoz, S., Lazagabaster, I. A., Alroy, J., & Evans, A. R. (2017). Inferring diet from dental morphology in terrestrial mammals. Methods in Ecology and Evolution, 8, 481–491. https://doi.org/10.1111/2041-210X.12691.
R Core Team. (2021). R: a language and environment for statistical computing. R Foundation for Statistical Computing.
Rambaut, A., Drummond, A. J., Xie, D., Baele, G., & Suchard, M. A. (2018). Posterior summarization in Bayesian phylogenetics using Tracer 1.7. Systematic Biology, 67, 901–904. https://doi.org/10.1093/sysbio/syy032.
Revell, L. J. (2012). Phytools: An R package for phylogenetic comparative biology (and other things). Methods in Ecology and Evolution, 3, 217–223. https://doi.org/10.1111/j.2041-210X.2011.00169.x.
Rohlf, F. J. (2006). A comment on phylogenetic correction. Evolution, 60, 1509–1515. https://doi.org/10.1111/j.0014-3820.2006.tb01229.x.
Segall, M., Houssin, C., Delapré, A., Cornette, R., Herrel, A., Milgram, J., Shahar, R., & Dumont, M. (2023). Armed to the teeth: The underestimated diversity in tooth shape in snakes and its relation to feeding behavior and diet. Ecology and Evolution, 13, e10011. https://doi.org/10.1002/ece3.10011.
Shetty, S., & Shine, R. (2002). Sexual divergence in diets and morphology in Fijian sea snakes Laticauda colubrina (Laticaudinae). Austral Ecology, 27, 77–84. https://doi.org/10.1046/j.1442-9993.2002.01161.x.
Shine, R. (1991). Intersexual dietary divergence and the evolution of sexual dimorphism in snakes. The American Naturalist, 138, 103–122. https://doi.org/10.1086/285207.
Suchard, M. A., Lemey, P., Baele, G., Ayres, D. L., Drummond, A. J., & Rambaut, A. (2018). Bayesian phylogenetic and phylodynamic data integration using BEAST 1.10. Virus Evolution, 4, vey016. https://doi.org/10.1093/ve/vey016.
Tonini, J. F. R., Beard, K. H., Ferreira, R. B., Jetz, W., & Pyron, R. A. (2016). Fully-sampled phylogenies of squamates reveal evolutionary patterns in threat status. Biological Conservation, 204, 23–31. https://doi.org/10.1016/j.biocon.2016.03.039.
Uetz, P., Freed, P., Aguilar, R., Reyes, F., & Hošek, J. (2022). The Reptile Database, http://www.reptile-database.org (accessed 30/3/2023).
Uyeda, J. C., Zenil-Ferguson, R., & Pennell, M. W. (2018). Rethinking phylogenetic comparative methods. Systematic Biology, 67, 1091–1109. https://doi.org/10.1093/sysbio/syy031.
Valenta, J. (2010). Venomous Snakes: Envenoming, Therapy. Nova Science Publishers.
Westeen, E. P., Durso, A. M., Grundler, M. C., Rabosky, D. L., & Rabosky, D., A. R (2020). What makes a fang? Phylogenetic and ecological controls on tooth evolution in rear-fanged snakes. BMC Evolutionary Biology, 20, 1–15. https://doi.org/10.1186/s12862-020-01645-0.
Zaher, H., Murphy, R. W., Arredondo, J. C., Graboski, R., Machado-Filho, P. R., Mahlow, K., Montingelli, G. G., Bottallo Quadros A., Orlov, N. L., Wilkinson, M., Zhang, Y-P., & Grazziotin F. G. (2019). Large-scale molecular phylogeny, morphology, divergence-time estimation, and the fossil record of advanced caenophidian snakes (Squamata: Serpentes). PLoS ONE, 14(5), e0216148. https://doi.org/10.1371/journal.pone.0216148
Zelditch, M. L., Swiderski, D. L., & Sheets, H. D. (2012). Geometric morphometrics for biologists: a primer. Academic Press.
Acknowledgements.
We thank Adelaide Microscopy and Microscopy Australia for access to the microCT scanning equipment at the University of Adelaide (Adelaide, Australia), A. Labridinis for the assistance provided, and Ammresh for microCT scanning some of the specimens; we are very grateful for the CT scans kindly provided upon request by A. Davis-Rabosky, G. Schneider and R. Nagesan at UMMZ, and by S. Kennedy-Gold and M. Aja at MCZ. We also thank all the contributors and curators on Morphorphosource.org for providing access to some of the data used in this study. We thank R. Foster and M. Hutchinson for assistance accessing specimens at the South Australian Museum. We are also grateful to M. Segall and an anonymous reviewer for their helpful comments.
Funding
Open Access funding enabled and organized by CAUL and its Member Institutions. This study was funded by the Australian Research Council as a Discovery Project Grant (DP200102328).
Author information
Authors and Affiliations
Contributions
AP designed the study, collected the data, analysed the data with input from ES and MYSL, prepared the figures, and wrote the first draft of the manuscript. All authors contributed to the interpretation of the results and the writing of the discussion. All authors read, edited, and approved the final version of the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Palci, A., Lee, M.S.Y., Crowe-Riddell, J.M. et al. Shape and Size Variation in Elapid Snake Fangs and the Effects of Phylogeny and Diet. Evol Biol 50, 476–487 (2023). https://doi.org/10.1007/s11692-023-09617-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11692-023-09617-0