Abstract
Within-plant variation in seed size may merely reflect developmental instability, or it may be adaptive in facilitating diversifying bet-hedging, that is, production of phenotypically diverse offspring when future environments are unpredictable. To test the latter hypothesis, we analyzed patterns of variation in seed size in 11 populations of the perennial vine Dalechampia scandens grown in a common greenhouse environment. We tested whether population differences in the mean and variation of seed size covaried with environmental predictability at two different timescales. We also tested whether within-plant variation in seed size was correlated with independent measures of floral developmental instability and increased under stressful conditions. Populations differed genetically in the amount of seed-size variation occurring among plants, among infructescences within plants, and among seeds within infructescences. Within-individual variation was not detectably correlated with measures of developmental instability and did not increase under stress, but it increased weakly with short-term environmental unpredictability of precipitation at the source-population site. These results support the hypothesis that greater variation in seed size is adaptive when environmental predictability is low.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Despite theoretical models suggesting the existence of an optimal size for propagules produced by an individual (Smith & Fretwell, 1974; Stearns, 1992; de Jong & Klinkhamer, 2005), seed size is surprisingly variable within plants (Herrera, 2009; Michaels et al., 1988; Obeso, 1993; Pélabon et al., 2015; Susko & Lovett-Doust, 2000; Vaughton & Ramsey, 1998). Within-plant variation in seed size may reflect deterministic or random positional effects in modular sessile organisms (Diggle, 1995; Herrera, 2009), or it may reflect developmental instability, that is, the inability of a genotype to produce seeds with a constant phenotype due to developmental noise (Nijhout & Davidowitz, 2003; Van Dongen, 2006). With a constant fitness optimum, such phenotypic variation in seed size is expected to decrease fitness by increasing adaptive imprecision (Hansen et al., 2006; Pélabon et al., 2012), and the resulting stabilizing selection is expected to canalize seed size against genetic and developmental variation (Silvertown, 1989). If the optimum changes stochastically or fluctuates, however, this phenotypic variation may become adaptive (Pélabon et al., 2012; Simons & Johnston, 1997; Tufto, 2015). Indeed, the production by a single individual of phenotypically variable offspring, each optimal for a different environmental condition, may increase the individual’s fitness by buffering variation in reproductive success and maximizing the long-term fitness. Such a strategy is referred to as diversifying bet-hedging (Lewontin & Cohen, 1969; Marshall et al., 2008; Philipi & Seger, 1989; Seger & Brockmann, 1987; Simons, 2009; Starrfelt & Kokko, 2012).
Studies of seed dormancy have provided some of the most convincing examples of diversifying bet-hedging. In some studies, population or species differences in the duration of dormancy or in the fraction of seeds germinating in each favorable season correlate with measures of environmental variation or unpredictability (Cohen, 1966; Clauss & Venable, 2000; Donohue et al., 2005; Gremer & Venable, 2014; Philipi, 1993; Tielborger et al., 2012; Venable, 2007). While variation in germination or dispersal behavior among seeds is sometimes associated with discrete polymorphism in seed size or shape (i.e. seed heteromorphism; Venable, 1985; Venable et al., 1987; Imbert, 2002), variation is often more cryptic and continuous. Accordingly, variation in seed dormancy or germination behavior is often associated with variation in seed size. Among species, time to germination generally increases with seed mass (Harel et al., 2011; Norden et al., 2009), while within species, several studies report that larger seeds germinate earlier, or are more likely to germinate than smaller ones (Biere, 1991; Simons & Johnston, 2000; Galloway, 2001; Pélabon et al., 2005, but see Susko & Lovett-Doust, 2000 for the opposite pattern). Furthermore, in seeds exhibiting dormancy, the time necessary to break dormancy and induce germination under favorable conditions sometimes increases with seed mass (Martins et al., 2019). Variation in seed size may thus provide a mechanism for producing seeds with different germination times, and diversifying bet-hedging could therefore represent an adaptive explanation for the unexpected within-individual and within-inflorescence variation in seed size often observed in angiosperms.
If within-plant variation in seed size represents diversifying bet-hedging, genotypes with more variable seed size should have higher long-term fitness under natural conditions (e.g. Donohue et al., 2005; Simons, 2011). This prediction is difficult to test directly in natural environments, especially for perennial species. An alternative approach is to test whether differences among species or populations in within-plant variation in seed size correlate with measures of variation and unpredictability of the environment experienced by those species or populations (e.g. Clauss & Venable, 2000; Scholl et al., 2020).
Here, using 11 populations of the Neotropical vine, Dalechampia scandens (Euphorbiaceae), we first assess whether population differences in within-individual variation in seed size is genetically determined by testing if populations grown and maintained in a common environment differ in the among-individual, within-individual and within-blossom components of variations in seed size. We then test whether average seed size and within-plant variation in seed size observed in each population correlate with the seasonality and unpredictability of the environment experienced historically by these populations. Finally, to understand further the nature of the within-plant variation in seed size, we test whether this variation correlates with other measures of developmental instability or increases under stressful conditions.
Methods
Study Organisms
Dalechampia scandens L. (s.l.) is a perennial vine that produces many blossoms (cluster of ca. ten staminate and three pistillate flowers that function together as a pollination unit; Fig. 1) during its life. A specialized gland-like structure associated with the staminate flowers produces resin that attracts resin-collecting bee pollinators (Armbruster, 1984, 1996; Webster & Webster, 1972). Each of the three pistillate flowers contains three ovules, resulting in a maximum of nine seeds per blossom (Armbruster, 1982). Mature seeds are dispersed by explosive shattering of the three-seeded capsules. All three capsules in a blossom (infructescence) develop and explode roughly simultaneously in association with withering of the involucral bracts and spreading of the pistillate sepals (Armbruster, 1982).
Illustration of D. scandens blossom inflorescence and trait measurements. The depicted blossom is in the first day of the bisexual phase with the first male flower open. The resin-producing gland is visible above the male flowers and the three stigmas of the three female flowers are below the cluster of male flowers. The measurement illustrated on the right are: GHr and GHl for gland height right and left, and SWr, SWc, SWl for stigma width, right, centre, and left.
Previous experiments have shown that neither seed size nor within-blossom variation in seed size is detectably affected by the number of fathers siring the clutch (Pélabon et al., 2015). Furthermore, within populations, seed size correlates positively with the duration of seed after-ripening required before germination, and among populations, the duration of the after-ripening period correlates positively with environmental seasonality (Martins et al., 2019). Once dormancy is broken, however, larger seeds have a higher probability of germinating (Pélabon et al., 2005). Furthermore, several studies of various populations have failed to detect a seed size-number trade-off at the within-blossom level (Hildesheim et al., 2020; Pélabon et al., 2015, 2016). The Dalechampia blossom is bilaterally symmetrical, thus allowing measurements of fluctuating asymmetry (FA, random differences in the size of an organ on each side of the symmetry axis) as an estimate of developmental instability (Pélabon et al., 2004a, b).
Study Populations and Data Collection
From 1998 to 2016, we collected seeds from several populations of D. scandens s.l. in Venezuela, Mexico (Bolstad et al., 2014) and Costa Rica (Opedal et al., 2016a). Most of these belong to the taxon previously referred to as ‘large-glanded’ D. scandens (Bolstad et al., 2014; Falahati-Anbaran et al., 2013, 2017). Seeds from each population were germinated in the greenhouse of the Department of Biology (Norwegian University of Science and Technology, NTNU, Trondheim, Norway) and individuals were maintained under similar conditions. We combined data on variation in seed size and developmental instability in blossom traits from 11 populations (See Table 1 for details). Seeds were produced by hand-pollination of blossoms from which male flowers had been removed before the bisexual phase (see e.g. Pélabon et al., 2015 for details). Pollination was ensured by brushing stigmas with one or two freshly opened male flowers from a designated pollen donor. Using empty teabags sealed around the pollinated blossoms, we collected the seeds after dehiscence. For each population, we obtained two measures of blossom developmental instability by estimating fluctuating asymmetry in the height of the resin-producing gland and in the width of the stigmas. All plants producing blossoms and seeds were grown entirely in the greenhouse, either from seeds collected in the wild or from seeds produced in the greenhouse (Table 1). All plants were maintained under similar conditions with a 13:11-h light/dark regime and the temperature set at 25 °C during the day and 23 °C at night. We watered the plants daily by flooding the tables with ca. 5 cm of water (except in the drought experiment; see below) and fertilized them weekly.
Drought Experiment
To test whether within-plant variation in seed size increased in stressful environments as expected for traits indicating developmental instability, we made new sets of crosses while exposing some of the plants from two populations to drought (Tulum: 21 plants in the dry environment and 10 in the benign/control environment and 16 and 10 for Tovar, respectively). More plants were allocated to the dry environment to compensate for blossom failures to set seeds. We followed the experimental design of Opedal et al. (2016b) and exposed plants to a dry treatment by carefully monitoring daily the plants and top-watering individual plants with 200 mL of water when they showed signs of drought stress (drooping leaves, indicating loss of turgor pressure). Plants in the benign environment were watered daily as described above. Plants were exposed to two weeks of drought conditions before starting the hand-pollination, and the treatment was maintained until all seeds were harvested. Pollination and seed collection were performed as described above.
Measurements
Seed size was assessed by measuring the seed diameter with digital callipers along the hilum of the seed. This measure of seed size was preferred over seed mass because the latter varies temporally due to water loss. The measures are strongly correlated though (r = 0.92, n = 410), with an allometric exponent between log(seed mass) as response variable and log(seed diameter) as predictor variable of β = 2.97 ± 0.04, allowing conversion of results between measures (Raunsgard et al., 2018). Aborted seeds were easily identified by their small size and a light grey seed coat, and they were excluded. Repeatability of seed diameter at the within-population level estimated from repeated measurements of 739 seeds was high (all r between 0.91 and 0.94).
Fluctuating asymmetry of the gland was measured as the difference in height between the left and right sides of the resin gland, and fluctuating asymmetry of the stigma was measured as the difference in the width of the tip of the left and right stigma (Fig. 1). Fluctuating asymmetry (FA) was calculated as the absolute value of the difference between the two sides FA =|L − R| for each trait. We did not conduct repeated measures to estimate the contribution of measurement error to FA in this study, but previous analyses have shown that measurement errors in gland height and stigma width in the Tulum and Tovar populations were sufficiently small to allow reliable estimates of fluctuating asymmetry for these traits (Pélabon et al., 2004a, b). Furthermore, we did not expect the amount of measurement error to differ among populations and thus compromise our comparison of fluctuating asymmetry across populations. In the following, we refer collectively to these two measures of within-individual variation as measures of developmental instability.
Environmental Predictability
Environmental predictability has two components, (i) the seasonality, which represents the regularity in the timing and magnitude of the variation in the average environmental factor, and (ii) the “colour” of the environmental noise, that is, the degree to which the environment is dissimilar between successive time points (Burgess & Marshall, 2014; Colwell, 1974; Marshall & Burgess, 2015). While seasonality is expected to generate selection on the average germination behaviour, such as, the length of the after-ripening time necessary to germinate and the average duration of dormancy, short-term unpredictability of the environment may affect selection on the variance of such traits. For tropical dry-forest plants such as Dalechampia, a highly seasonal environment will be characterized by a marked and prolonged dry season during which seeds will encounter unfavourable conditions for germination and establishment. In such environments, mature seeds are expected to delay their germination so as to avoid germinating before the dry season. We thus expect seeds from population occurring in highly seasonal environments to have evolved long duration of after-ripening (Martins et al., 2019). If seed size is correlated with the duration of after-ripening, population average seed size should covary with seasonality. Environments characterized by short-term unpredictability should favour variation in germination behaviour, and we expect variance in seed characteristics to be higher in such environments.
Although germination cues may include specific interactions between light, moisture, and other environmental factors, we assumed that moisture was the major factor limiting seed germination, and we considered here seasonality and unpredictability in precipitation patterns as the environmental characteristics likely to affect variation in seed size. Using monthly precipitation data for the period 1901–2011 extracted from the CRU TS3.10 dataset (Harris et al., 2014), we calculated for each population two indices representing environmental seasonality and unpredictability. We first decomposed the time series of monthly precipitation into a seasonal and a random component using the R-function decompose that performed a seasonal decomposition of the time series with moving averages. We then mean-scaled the seasonal and the random components by the square of the average monthly precipitation over the whole time series. We considered the mean-scaled seasonal variance as an index of seasonality (higher values indicating stronger seasonality), and the mean-scaled random variance as an index of short-term environmental unpredictability (higher values indicating higher unpredictability).
Statistical Analyses
Population Differences in Patterns of Variation
To test whether populations differ in their patterns of variation in seed size, we compared two mixed-effect models fitted with the nlme R-package (Pinheiro et al., 2019). Both models had seed size as response variable, population as predictor variable and blossom nested within individual identity as random factors. In the first model, the structure of the random variance was similar across populations while in the second model, the structure of the random variance could differ across populations using the varIdent function (Zuur et al., 2009). Models were fitted with restricted maximum likelihood (REML) and compared using the Akaike information criterion (AIC).
We then estimated the different components of the variation in seed size for each population by fitting mixed-effect models in a Bayesian framework with the MCMCglmm R-package (Hadfield, 2010). We chose the Bayesian approach in order to obtain highest posterior density (HPD) intervals for the components of the random variance. For each population, the model included seed size as response variable, an intercept as predictor variable and blossom identity nested within plant identity as random effects. As priors for the models, we used zero-mean Gaussian distributions with very large variances (108) for the fixed effect, scaled F distributions where the variance/1000 was F1,1 distributed for the variance parameters, and inverse-Wishart distributed for the residuals (Hadfield, 2010). These models ran for 260,000 MCMC iterations with a burn-in phase of 10,000 and a thinning interval of 250 iterations for a total of 1000 samples from the posterior distribution. For two populations, Bacalar and Graciano Sánches, we had too few replicated crosses per individual to reliably estimate the among-blossom variance. For these two populations we only estimated the among-individual and within-blossom variances components.
To test whether developmental instability differed among populations, we compared mixed-effect models where FA in gland height or stigma width were the response variables, population the predictor variable, and individual identity a random factor. To comply with the requirement of normally distributed residuals, FA measures were cubic-root transformed (see Pélabon et al., 2004a for a discussion on the choice of the transformation). Models were fitted with maximum likelihood (ML) and compared using AIC. Population-mean fluctuating asymmetries and their 95% confidence interval were estimated by non-parametric bootstrapping on non-transformed data, by resampling at the level of the individual blossom.
The relationship between developmental instability and trait size may provide valuable insight into the process generating developmental noise and inform about the necessity to correct measures of variation for differences in trait mean when comparing levels of variation across populations (Pélabon et al., 2020; Soulé, 1982). We thus investigated whether within-individual variation in seed size and FA measures covaried with trait size within and among populations. We also tested whether within-blossom variation in seed size was correlated with measures of developmental instability also collected at the blossom level and whether these fluctuating asymmetry measures were correlated with each other.
Effect of the Drought Treatment on Seed Size and Variation in Seed Size
To test the effect of drought on mean and variance in seed size, we used the varIdent function in the nlme R-package to compare mixed-effect models allowing or not the random variance to differ among treatments. For each population, we fitted models where seed size was the response variable, treatment the predictor variable and blossom identity nested with plant identity were random factors. Models were fitted with REML and compared using AIC.
Relationship Between Variation in Seed Size and Environmental Characteristics
Among-plant variation in seed size may result from micro-environmental variation, (additive) genetic variation, variation in maternal effects, and their interactions. Within-plant variation in seed size on the other hand, may result from micro-environmental variation (e.g. within plant shading) as well as differences in energy allocation, among blossoms due to differences in the position of the blossoms relative to the main stem, within blossoms due to the positioning of the flower in the blossom (i.e. lateral vs. terminal flowers), or within flower (Diggle, 1995, 2014; Herrera, 2009). In our study, the two latter levels of variation are confounded because we could not distinguish the flower from which the seeds originated after explosive dehiscence. Fluctuating selection is expected to favour phenotypic variation both within and among individuals. However, other factors such as breeding system and population size may also affect genetic variation among individuals (Clo et al., 2019; Willi et al., 2006). Therefore, only within-individual variation in seed size is expected to consistently reflect the strength of stabilizing selection on seed size that may covary with environmental predictability.
Assuming that the after-ripening duration necessary for seeds to germinate is positively correlated with seed size, we first tested if seed size increases with environmental seasonality (Martins et al., 2019). We also tested whether variation in seed size increases with short-term environmental unpredictability as suggested by the diversifying bet-hedging hypothesis. For both analyses, we used least-squares regression, weighted by the inverse of the squared standard error of each response variable data point. All statistical analyses were performed with R version 4.0.2 (R Core Team, 2020).
Results
Population Differences in Patterns of Variation
Although the size variation of the Dalechampia seeds obtained under greenhouse conditions was relatively small, with a maximum within-population coefficient of variation (CV) in seed diameter of 8%, populations differed both in the magnitude of variation in seed size and how this variation was distributed across individuals, blossoms and seeds (Table 2). These differences in patterns of variation were statistically supported by the better fit of the mixed-effect model that allowed population differences in the partitioning of the variance (∆AIC = 395.78; Table S1). Except for the Tovar and Palo Verde populations, more than half of the variation in seed size was expressed at the within-individual level. In the Tulum and Martinez de la Torre populations, as much as 90% of the variation in seed size occurred at the within-individual level. Populations also differed in the proportion of variance in seed size expressed at the within-blossom level which ranged from 6 to 25% of the total within-population variance (Table 2). Analyses performed on natural-log-transformed data yielded qualitatively similar results (not shown).
Among populations, there were only weak, if any, correlations between mean seed size and the different components of variance in seed size (total variance r = 0.09, among-blossom variance r = 0.14, within-blossom variance r = 0.25, n = 11, p > 0.44 for all tests). Within populations, however, within-blossom variation in seed size decreased with increasing blossom-mean seed size, and this pattern was similar across all populations (Fig. 2A; Table S1). On a proportional scale, the slope of the regression of Log(SD) on ln(mean seed size) common to all populations is b = − 2.49 (± 0.33). It indicates that a 1% increase in mean seed size is expected to generate a 2.5% decrease in the within-blossom standard deviation in seed size. If this negative relationship simply reflects a decrease in the mean seed size when the variance increases, it should vanish when we consider instead the maximum seed size produced by the blossoms as predictor variable. The negative relationship, b = − 0.73 (± 0.36), observed in this new analysis with maximum seed size confirmed that within-blossom variation in seed size decreases with an increasing average seed size.
Relationship between trait mean and within-blossom variation for A seed size, B gland height and C stigma width. The within blossom variation in seed size is measured by the standard deviation SD in seed diameter, and the within-blossom variation for the two other traits is measured by their fluctuating asymmetry. Grey dots represent individual blossom measurements, black dots represent population means. Black lines represent the regressions of the within-blossom variation on the blossom mean for each population. These regressions are presented when statistically supported and parameter estimates are obtained from models fitted on data expressed on the original scale (mm)
The two measures of developmental instability differed among populations (Table 3; Table S1) but were only weakly correlated with each other across populations (r = 0.22, p = 0.51, n = 11). Variation in fluctuating asymmetry in gland height was largely independent of variation in mean gland height within and among populations (Fig. 2B; Table S1, among population r = 0.20, p = 0.55, n = 11), while fluctuating asymmetry in stigma width increased with the mean stigma width within population (Fig. 2C; Table S1, regression slope of FA1/3 on mean style width: b = 0.023 ± 0.0067), but not among populations (r = 0.21, p = 0.52, n = 11). Neither fluctuating asymmetry measure was detectably correlated with the within-blossom variation in seed size (FA gland height: r = − 0.33, p = 0.31; FA in stigma width: r = − 0.24, p = 0.47, n = 11) (see Table 4).
Effect of Drought Treatment on Seed Size and Variation in Seed Size
When plants from Tulum and Tovar were exposed to drought, the mean seed size decreased by 1.5% in Tulum (from 3.90 ± 0.05 to 3.84 ± 0.03 mm), and 3% in Tovar (from 3.12 ± 0.04 to 3.02 ± 0.03 mm; see Table S1 for statistical tests). However, the treatment did not detectably affect the within-individual and within-blossom variation in seed size (Table 4 and Table S1).
Relationship Between Variation in Seed Size, Seasonality and Environmental Unpredictability
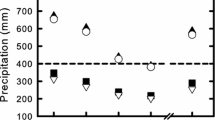
Among populations, mean seed size tended to increase with environmental seasonality, as expected, although this relationship had no statistical support (Fig. 3A). As predicted by the diversifying bet-hedging hypothesis, within-plant variation in seed size (sum of the within and among-blossom variation) increased with increasing unpredictability of the environment (Fig. 3B), although this result was only marginally statistically significant. To interpret this relationship, recall that an unpredictability index of 0.20, as observed for the Puerto Morelos population, corresponds to a CV in unpredictable precipitation of \(\sqrt {0.20} \approx 0.45\), that is, 45% of the average monthly precipitation, and an index of 0.35, as observed for the Martinez de la Torre population, corresponds to ≈ 60% of unpredictable monthly precipitation. Across this range of environmental unpredictability, the increase in within-individual standard deviation in seed size was ≈ 0.03 mm for an average within-individual standard deviation of 0.14 mm (average across nine populations). Although this increase represents an important contribution to the within-plant variation in seed size, this result must be considered with caution considering the relatively small sample size and the uncertainty of the estimate. Note that we found no relationship between environmental unpredictability and the within blossom variation in seed size [100 × SDwb = 7.35 (± 4.31) − 0.67 (± 15.51) × unpredictability].
Relationship between A mean seed size and environmental seasonality and B within-plant variation in seed size and environmental unpredictability for nine populations of D. scandens. Seasonality and unpredictability are defined as the seasonal and the residual variance components of mean-scaled monthly precipitation, where mean scaling is achieved by dividing by the grand mean squared. The models for the weighted regressions are for A: mean seed size = 3.79 (± 0.18) + 0.63 (± 0.50) × seasonality, and for B: 100 × standard deviation of seed size = 8.62 (± 3.31) + 21.78 (± 12.65) × unpredictability. Note that the imprecision of both environmental characteristics may generate attenuation of the regression slope particularly for environmental unpredictability. SD standard deviation
Discussion
Seed size has traditionally been considered as a canalized trait (Harper et al., 1970; Silvertown, 1989), yet many studies have reported variation in seed size among and within individuals (Herrera, 2009; Michaels et al., 1988; Obeso, 1993; Pélabon et al., 2015, 2016; Susko & Lovett-Doust, 2000; Turnbull et al., 2006; Vaughton & Ramsey, 1998). Our study also shows variation in seed size, albeit limited. The average CV in seed diameter of ca. 5% (range 2–8%) reflects much less proportional size variation than observed for other blossom traits. For example, the average CV for gland height and style width estimated in our 11 populations reach 13% and 15%, respectively. The canalization of seed size against environmental variation is further demonstrated by the limited effect of drought on this trait. Indeed, the mean seed diameter decreases by 1–3% under dry conditions, while Opedal et al. (2016b) observed a 25% decrease in the size of the resin gland under similar stressful conditions. Halpern (2005) also reported no detectable effect of water availability on seed size of Lupinus perennis. These results suggest that seed size is under stabilizing or canalizing selection and that its variation is partly buffered from variation in blossom size, as further demonstrated by the weak correlation observed between blossom size and seed size (Pélabon et al., 2015). Our results also confirm that populations differed genetically in the amount of variation in seed size, and particularly in the distribution of this variation among and within plants. Among the study populations, the proportion of the variance in seed size expressed at the within-plant level ranged from 40 to 90%.
Population differences in the among-individual variation in seed size exhibited in a common environment may result from differences in additive genetic variation, maternal genetic effects (Galloway et al., 2009) or differences in maternal environmental effects generated by population specific gene by environment interactions, with some populations being more canalized than others against (micro) environmental variation. The current study does not allow us to distinguish these sources of variation, yet we note that previous experiments (Pélabon et al., 2015, 2016) have reported very limited additive genetic variation in seed mass in the Tulum population for which we observed the lowest among-individual variation in this study. The current results suggest that some populations may harbour more genetic variation in seed size than observed in the Tulum population.
Within-plant variation in seed size was dominated by the variation among blossoms. Differences among populations in this component of variation may originate from differences in the within-plant variation in blossom size generated by positional effects and differences in the architecture of the plants. For example, plants from the Tovar population produce many blossoms of relatively constant size (within-plant CV in gland height = 8.6%), while plants from the Tulum population produce fewer blossom that are more variable in size (within-plant CV in gland height was estimated at = 15.4%). We could not fully test the hypothesis that population differences in the among-blossom variation in seed size resulted from differences in the variation in blossom size because the number of blossoms measured per plant in the other populations was too low to accurately estimate this component of the variance in blossom size. We note, however, that the buffering of the variation in seed size from variation in blossom size limits the proportion of among-blossom variation in seed size that can be explained by variation in blossom size. This leaves open the possibility that developmental instability explains part of the among-blossom variation in seed size.
Similarly, within-blossom variation in seed size may reflect differences in resource allocation due to positional effects among (lateral vs. terminal flowers) and within flowers, or developmental instability. The decrease in within-blossom variation in seed size with increasing blossom-mean seed size is difficult to explain, especially because, contrary to the observation by Turnbull et al. (2006) in Ceratonia siliqua (carob), it reflects a decrease in absolute variance as the mean seed size increases. This negative relationship was similar across all populations and could result from both architectural (positional effects) or random (developmental noise) variation whose effects on seed size are more important for smaller seeds.
Thus, within- and among-blossom variation in seed size may result either from positional effects or developmental instability, that is, from either deterministic or random variation of the development. The absence of detectable correlation between the within-blossom variation in seed size and two measures of blossom developmental instability do not support the hypothesis of a developmental instability origin of this seed-size variation, although measures of developmental instability are rarely correlated within organisms (Polak et al., 2003). This hypothesis is further contradicted by the lack of detectable effect of drought on variation in seed size. Therefore, we speculate that the within-plant variation in seed size observed in each population mostly results from deterministic variation in the development related to the architecture of the plants specific to each population and thus is genetically controlled.
Bet-hedging theory posits that in unpredictable environments, individuals could increase their long-term fitness by producing variable offspring. Simons and Johnston (1997) further suggested that the production of variable offspring may be achieved via developmental instability. The positive relationship between within-individual variation in seed size and environmental unpredictability observed in our study provides some supports to the bet-hedging hypothesis. However, in contrast to the hypothesis by Simons and Johnston (1997), we suggest that population differences in phenotypic variation in seed size do not reflect differences in developmental stability per se, but instead reflect differences in the strength of canalizing selection on the variation in seed size that results mostly from deterministic (architectural) variation of the development. Differences in the distribution of the within-individual variance in seed size further suggest that populations can achieve the suitable level of variation for a given level of environmental unpredictability via different combinations of within- and among-blossom variation, depending on the constraints imposed by the architecture of the plants. We further speculate that this method of generating phenotypic variation in seed size may be more evolvable than developmental instability and may allow populations to respond more rapidly to changes in environmental unpredictability. Indeed, variation in plant architecture is readily observed among populations (see above) but also among species of the genus Dalechampia as illustrated in Pax and Hoffmann (1919).
Assuming that seasonality in precipitation is an important source of selection on seed dormancy and germination behaviour, the weak tendency for seed size to increase with seasonality tends to support the conclusion that seed size in D. scandens is correlated with dormancy and germination behaviour (Martins et al., 2019). However, even taken at face value, this effect remains limited, with an increase in seed size of ca. 0.25 mm (just above 6% of the average seed size) over the whole range of seasonality. We also note that factors other than precipitation may affect the favourability of the environment for germination and seedling establishment (e.g. light, Chavez-Pesqueira & Nunez-Farfan, 2016; Michaels et al., 1988) and generate covariance between seed size and seasonality. In Dalechampia, such a covariance may result from the ability of seedlings to establish and elongate before the canopy closes with the advancing wet season. If seedling growth rate is correlated with seed size as is often observed (Leishman et al., 2000), we may expect seed size to covary with seasonality because changes in the canopy are expected to be more important in seasonal environment. Exact predictions are difficult to make, however, because both positive and negative relationships have been observed between seed size and seedling growth rate (Jurado & Westoby, 1992; Leishman et al., 2000; Wulff, 1986).
Quantifying environmental unpredictability is not straightforward, and it is particularly important to define the timescale at which unpredictability affects the fitness of the organism. Here, we considered that short-term unpredictability should affect the time-to-germination once dormancy is broken. With the data available, we chose to estimate short-term unpredictability as the proportional variation in precipitation once the variation due to seasonality has been accounted for. This remains a crude measure, particularly because it includes some variation that may not affect the probability of germination. Furthermore, variation in seed size may also be affected by the level of unpredictability in the light regime or other factors encountered by the different populations.
Overall, our study provides some support for the bet-hedging hypothesis, which suggests that unpredictable environments favour variation and weaken canalizing selection. Yet, following Marshall and Burgess (2015), we suggest that progress in the understanding of bet-hedging strategies and adaptive within-plant variation in seed characteristics requires better quantification of environmental unpredictability.
Data Availability
Data and script for the analysis and the figures are available as supplementary material.
References
Armbruster, W. S. (1982). Seed production and dispersal in Dalechampia (Euphorbiaceae)—Divergent patterns and ecological consequences. American Journal of Botany, 69, 1429–1440.
Armbruster, W. S. (1984). The role of resin in angiosperm pollination—Ecological and chemical considerations. American Journal of Botany, 71, 1149–1160.
Armbruster, W. S. (1996). Evolution of floral morphology and function: An integrative approach to adaptation, constraint and compromise in Dalechampia (Euphorbiaceae). In D. G. Lloyd & S. C. H. Barrett (Eds.), Floral biology: Studies of floral evolution in animal-pollinated plants (pp. 241–272). Chapman and Hall.
Biere, A. (1991). Parental effects in Lychnis floscuculi. I: Seed size, germination and seedling performance in a controlled environment. Journal of Evolutionary Biology, 4, 447–465.
Bolstad, G. H., Hansen, T. F., Pélabon, C., Falahati-Anbaran, M., Perez-Barrales, R., & Armbruster, W. S. (2014). Genetic constraints predict evolutionary divergence in Dalechampia blossoms. Philosophical Transaction of the Royal Society, London B, 369, 1649.
Burgess, S. C., & Marshall, D. J. (2014). Adaptive parental effects: The importance of estimating environmental predictability and offspring fitness appropriately. Oikos, 123, 769–776.
Chavez-Pesqueira, M., & Nunez-Farfan, J. (2016). Habitat fragmentation changes the adaptive value of seed mass for the establishment of a tropical canopy tree. Biotropica, 48, 628–637.
Clauss, M., & Venable, D. L. (2000). Seed germination in desert annuals: An empirical test of adaptive bet hedging. American Naturalist, 155, 168–186.
Clo, J., Gay, L., & Ronfort, J. (2019). How does selfing affect the genetic variance of quantitative traits? An updated meta-analysis on empirical results in angiosperm species. Evolution, 73, 1578–1590.
Cohen, D. (1966). Optimizing reproduction in a randomly varying environment. Journal of Theoretical Biology, 12, 119–129.
Colwell, R. K. (1974). Predictability, constancy, and contingency of periodic phenomena. Ecology, 55, 1148–1153.
de Jong, T., & Klinkhamer, P. (2005). Evolutionary ecology of plant reproductive strategies. Cambridge University Press.
Diggle, P. K. (1995). Architectural effects and the interpretation of patterns of fruit and seed development. Annual Review of Ecology Evolution and Systematics, 26, 531–552.
Diggle, P. K. (2014). Modularity and intra-floral integration in metameric organisms: Plants are more than the sum of their parts. Philosophical Transaction of the Royal Society, London b., 369, 20130253.
Donohue, K., Dorn, L., Griffith, C., Kim, E., Aguilera, A., Polisetty, C. R., & Schmitt, J. (2005). The evolutionary ecology of seed germination of Arabidopsis thaliana: Variable natural selection on germination timing. Evolution, 59, 758–770.
Falahati-Anbaran, M., Stenoien, H. K., Bolstad, G. H., Hansen, T. F., Perez-Barrales, R., Armbruster, W. S., & Pélabon, C. (2017). Novel microsatellite markers for Dalechampia scandens (Euphorbiaceae) and closely related taxa: Application to studying a species complex. Plant Species Biology, 32, 179–186.
Falahati-Anbaran, M., Stenoien, H. K., Pélabon, C., Bolstad, G. H., Perez-Barrales, R., Hansen, T. F., & Armbruster, W. S. (2013). Development of microsatellite markers for the Neotropical vine Dalechampia Scandens (Euphorbiaceae). Applied Plant Science. https://doi.org/10.3732/apps.1200492
Galloway, L. F. (2001). The effect of maternal and paternal environments on seed characters in the herbaceous plant Campanula americana (Campanulaceae). American Journal of Botany, 88, 832–840.
Galloway, L. F., Etterson, J. R., & McGlothlin, J. W. (2009). Contribution of direct and maternal genetic effects to life-history evolution. New Phytologist, 183, 826–838.
Gremer, J. R., & Venable, D. L. (2014). Bet hedging in desert winter annual plants: Optimal germination strategies in a variable environment. Ecology Letters, 17, 380–387.
Hadfield, J. D. (2010). Methods for multi-response generalized linear mixed models: The MCMCglmm R package. Journal of Statistical Software, 33, 1–22.
Halpern, S. L. (2005). Sources and consequences of seed size variation in Lupinus perennis (Fabaceae): Adaptive and non-adaptive hypotheses. American Journal of Botany, 92, 205–213.
Hansen, T. F., Carter, A. J. R., & Pélabon, C. (2006). On adaptive accuracy and precision in natural populations. American Naturalist, 168, 168–181.
Harel, D., Holzapfel, C., & Sternberg, M. (2011). Seed mass and dormancy of annual plant populations and communities decreases with aridity and rainfall predictability. Basic and Applied Ecology., 12, 674–684.
Harper, J. L., Lovell, P. H., & Moore, K. G. (1970). The shapes and sizes of seeds. Annual Review of Ecology and Systematics, 1(1), 327–356.
Harris, I., Jones, P., Osborn, T., & Lister, D. (2014). Updated high-resolution grids of monthly climatic observations–the CRU TS3. 10 Dataset. International Journal of Climatology, 34, 623–642.
Herrera, C. M. (2009). Multiplicity in unity: Plant subindividual variation and interactions with animals. Chicago University Press.
Hildesheim, L. S., Opedal, Ø. H., Armbruster, W. S., & Pélabon, C. (2020). Quantitative and qualitative consequences of reduced pollen loads in a mixed-mating plant. Ecology and Evolution, 9, 14253–14260.
Imbert, E. (2002). Ecological consequences and ontogeny of seed heteromorphism. Perspectives Plant Ecology, Evolution and Systematics, 5, 13–36.
Jurado, E., & Westoby, M. (1992). Seedling growth in relation to seed size among species of arid Australia. Journal of Ecology, 80, 407–416.
Leishman, M. R., Wright, I. J., Moles, A. T., & Westoby, M. (2000). The evolutionary ecology of seed size. In M. Fenner (Ed.), Seeds: The ecology of regeneration in plant communities (pp. 31–57). CABI publishing.
Lewontin, R. C., & Cohen, D. (1969). On population growth in a randomly varying environment. Proceedings of the National Academy of Science, 62, 1056–1060.
Marshall, D. J., Bonduriansky, R., & Bussiere, L. F. (2008). Offspring size variation within broods as a bet-hedging strategy in unpredictable environments. Ecology, 89, 2506–2517.
Marshall, D. J., & Burgess, S. C. (2015). Deconstructing environmental predictability: Seasonality, environmental colour and the biogeography of marine life histories. Ecology Letters, 18, 174–181.
Martins, A. A., Opedal, Ø. H., Armbruster, W. S., & Pélabon, C. (2019). Rainfall seasonality predicts the germination behavior of a tropical dry-forest vine. Ecology and Evolution, 9, 5196–5205.
Michaels, H. J., Benner, B., Hartgerink, A. P., Lee, T. D., Rice, S., Willson, M. F., & Bertin, R. I. (1988). Seed size variation: Magnitude, distribution, and ecological correlates. Evolutionary Ecology, 2, 157–166.
Nijhout, F. H., & Davidowitz, G. (2003). Developmental perspectives on phenotypic variation, canalization, and fluctuating asymmetry (pp. 3–13). Oxford University Press.
Norden, N., Daws, M. I., Antoine, C., Gonzalez, M. A., Garwood, N. C., & Chave, J. (2009). The relationship between seed mass and mean time to germination for 1037 tree species across five tropical forests. Functional Ecology, 23, 203–210.
Obeso, J. R. (1993). Seed mass variation in the perennial herb Asphodelus albus—Sources of variation and position effect. Oecologia, 93, 571–575.
Opedal, O. H., Albertsen, E., Armbruster, W. S., Perez-Barrales, R., Falahati-Anbaran, M., & Pélabon, C. (2016a). Evolutionary consequences of ecological factors: Pollinator reliability predicts mating-system traits of a perennial plant. Ecology Letters, 19, 1486–1495.
Opedal, O. H., Listemann, J., Albertsen, E., Armbruster, W. S., & Pélabon, C. (2016b). Multiple effects of drought on pollination and mating-system traits in Dalechampia scandens. International Journal of Plant Sciences, 177, 682–693.
Pax, F., & K. Hoffmann. (1919). Euphorbiaceae-Dalechampieae. Das Pflanzenreich IV. 147. XII (Heft 68): 1–59.
Pélabon, C., Albertsen, E., Falahati-Anbaran, M., Wright, J., & Armbruster, W. S. (2015). Does multiple paternity affect seed mass in angiosperms? An experimental test in Dalechampia scandens. Journal of Evolutionary Biology, 28, 1719–1733.
Pélabon, C., Armbruster, W. S., Hansen, T. F., Bolstad, G. H., & Pérez-Barrales, R. (2012). Adaptive accuracy and adaptive landscapes. In E. Svensson & R. Calsbeek (Eds.), The adaptive landscape in evolutionary biology (pp. 150–168). Oxford University Press.
Pélabon, C., Carlson, M. L., Hansen, T. F., & Armbruster, W. S. (2005). Effects of crossing distance on offspring fitness and developmental stability in Dalechampia scandens (Euphorbiaceae). American Journal of Botany, 92, 842–851.
Pélabon, C., Hansen, T. F., Carlson, M. L., & Armbruster, W. S. (2004a). Variational and genetic properties of developmental stability in Dalechampia scandens. Evolution, 58, 504–514.
Pélabon, C., Hansen, T. F., Carlson, M. L., Yoccoz, N. G., & Armbruster, W. S. (2004b). Consequences of inter-population crosses on developmental stability and canalization of floral traits in Dalechampia scandens (Euphorbiaceae). Journal of Evolutionary Biology, 17, 19–32.
Pélabon, C., Hennet, L., Bolstad, G. H., Albertsen, E., Opedal, Ø. H., Ekrem, R. K., & Armbruster, W. S. (2016). Does stronger pollen competition improve offspring fitness when pollen load does not vary? American Journal of Botany, 103, 522–531.
Pélabon, C., Hilde, C. H., Einum, S., & Gamelon, M. (2020). On the use of the coefficient of variation to quantify and compare trait variation. Evolution Letters, 4, 180–188.
Philippi, T. (1993). Bet-hedging germination of desert annuals: Variation among populations and maternal effects in Lepidium lasiocarpum. American Naturalist, 142, 488–507.
Philippi, T., & Seger, J. (1989). Hedging one’s evolutionary bets, revisited. Trends in Ecology and Evolution, 4, 41–44.
Pinheiro, J., Bates, D., DebRoy, S., Sarkar, D., Heisterkamp, S., Van Willigen, B., & Maintainer, R. (2017). Package ‘nlme’. In Linear and nonlinear mixed effects models, version, 3(1)
Polak, M., Møller, A. P., Gangestad, S. W., Kroeger, D. E., Manning, J. T., & Thornhill, R. (2003). Does an individual asymmetry parameter exist? A meta-analysis. In M. Polak (Ed.), Developmental instability causes and consequences. Oxford University Press.
Raunsgard, A., Opedal, Ø. H., Ekrem, R. K., Wright, J., Bolstad, G. H., Armbruster, W. S., Pélabon, C. (2018). Intersexual conflict over seed size is stronger in more outcrossed populations of a mixed-mating plant. Proceedings of the National Academy of Sciences, 115, 11561–11566.
R Core Team. (2020). R: A language and environment for statistical computing. R Foundation for Statistical Computing.
Scholl, J. P., Calle, L., Miller, N., & Venable, D. L. (2020). Offspring polymorphism and bet hedging: A large-scale, phylogenetic analysis. Ecology Letters, 23, 1223–1231.
Seger, J., & Brockmann, H. J. (1987). What is bet hedging? In P. H. Harvey & L. Partridge (Eds.), Oxford surveys in evolutionary biology (pp. 182–211). Oxford University Press.
Silvertown, J. (1989). The paradox of seed size and adaptation. Trends in Ecology and Evolution, 4, 24–26.
Simons, A. M. (2009). Fluctuating natural selection accounts for the evolution of diversification bet hedging. Proceedings of the Royal Society B: Biological Sciences, 276, 1987–1992.
Simons, A. M. (2011). Modes of response to environmental change and the elusive empirical evidence for bet hedging. Proceedings of the Royal Society B: Biological Sciences, 278, 1601–1609.
Simons, A. M., & Johnston, M. O. (1997). Developmental instability as a bet-hedging strategy. Oikos, 80, 401–406.
Simons, A. M., & Johnston, M. O. (2000). Variation in seed traits of Lobelia inflata (Campanulaceae): Sources and fitness consequences. American Journal of Botany, 87, 124–132.
Smith, C. C., & Fretwell, S. D. (1974). Optimal balance between size and number of offspring. American Naturalist, 108, 499–506.
Soulé, M. E. (1982). Allomeric variation. 1. The theory and some consequences. American Naturalist, 120, 751–764.
Starrfelt, J., & Kokko, H. (2012). Bet-hedging—A triple trade-off between means, variances and correlations. Biological Reviews, 87, 742–755.
Stearns, S. C. (1992). The evolution of life histories. Oxford University Press.
Susko, D. J., & Lovett-Doust, L. (2000). Patterns of seed mass variation and their effects on seedling traits in Alliaria petiolata (Brassicaceae). American Journal of Botany, 87, 56–66.
Tielborger, K., Petru, M., & Lampei, C. (2012). Bet-hedging germination in annual plants: A sound empirical test of the theoretical foundations. Oikos, 121, 1860–1868.
Tufto, J. (2015). Genetic evolution, plasticity, and bet-hedging as adaptive responses to temporally autocorrelated fluctuating selection: A quantitative genetic model. Evolution, 69, 2034–2049.
Turnbull, L. A., Santamaria, L., Martorell, T., Rallo, J., & Hector, A. (2006). Seed size variability: From carob to carats. Biology Letters, 2, 397–400.
Van Dongen, S. (2006). Fluctuating asymmetry and developmental instability in evolutionary biology: Past, present and future. Journal of Evolutionary Biology, 19, 1727–1743.
Vaughton, G., & Ramsey, M. (1998). Sources and consequences of seed mass variation in Banksia marginata (Proteaceae). Journal of Ecology, 86, 563–573.
Venable, D. L. (1985). The evolutionary ecology of seed heteromorphism. American Naturalist, 126, 577–595.
Venable, D. L. (2007). Bet hedging in a guild of desert annuals. Ecology, 88, 1086–1090.
Venable, D. L., Burquez, A., Corral, G., Morales, E., & Espinosa, F. (1987). The ecology of seed heteromorphism in Heterosperma pinnatum in Central Mexico. Ecology, 68, 65–76.
Webster, G. L., & Webster, B. D. (1972). The morphology and relationships of Dalechampia scandens (Euphorbiaceae). American Journal of Botany, 59, 573–586.
Willi, Y., Van Buskirk, J., & Hoffmann, A. A. (2006). Limits to the adaptive potential of small populations. Annual Review of Ecology Evolution and Systematics, 37, 433–458.
Wulff, R. D. (1986). Seed size variation in Desmodium paniculatum: II. Effects on seedling growth and physiological performance. Journal of Ecology, 74, 99–114.
Zuur, A., Ieno, E. N., Walker, N., Saveliev, A. A., & Smith, G. M. (2009). Mixed effects models and extensions in ecology with R. Springer.
Acknowledgements
We thank Grete Rakvaag for taking care of the plants during the experiments.
Funding
Open access funding provided by NTNU Norwegian University of Science and Technology (incl St. Olavs Hospital - Trondheim University Hospital). This work was partly supported by the Research Council of Norway through its Centre of Excellence funding scheme, project no. 223257 and NTNU.
Author information
Authors and Affiliations
Contributions
CP initiated the study. FDG, ØHO, GHB, CP and AR collected the data. CP performed the statistical analyses and wrote the manuscript with contribution from all coauthors.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare having no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Pélabon, C., De Giorgi, F., Opedal, Ø.H. et al. Is There More to Within-plant Variation in Seed Size than Developmental Noise?. Evol Biol 48, 366–377 (2021). https://doi.org/10.1007/s11692-021-09544-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11692-021-09544-y