Abstract
The transition to asexual reproduction is frequent and widespread across the tree of life and constitutes a major life history change. Without sexual reproduction, selection on sexually selected traits is expected to be weaker or absent, allowing the decay and ultimately loss of sexual traits. In this study, we applied an experimental approach to investigate the decay of reproductive traits under asexuality in two asexual populations of the springtail Folsomia candida. Specifically, we compared several key male sexual traits of a sexual population and two distinct parthenogenetic lines. To allow direct comparisons between sexual and asexual individuals we first determined a suite of life history characteristics in the sexual F. candida population, which performs an indirect transfer of sperm packages (spermatophores).To investigate the decay of male sexual traits under asexuality we measured the size of spermatophores, quantified the amount of sperm DNA material, and tested spermatophore attractiveness to females in all three populations. The amount of sperm DNA material in the sperm droplets and the attractiveness of spermatophores were lower in the asexual lines compared to the sexual population. However, the two asexual lines differed in the extent of decay of these traits. Our results are consistent with predictions from neutral mutation accumulation theory, and thus suggest this to be the main evolutionary process underlying the decay of male traits in F. candida.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Loss of traits plays an important role in the major transitions in evolution and is the focus of a large body of literature (see e.g., Fong et al. 1995; Lahti et al. 2009; Ellers et al. 2012). A shift in the reproductive mode of an organism (e.g., loss of sexual reproduction) can alter the direction and magnitude of selection pressures on traits that no longer contribute to individual fitness, such as sex-specific traits. In the transition from sexuality to parthenogenesis, a form of asexual reproduction that gives raise to offspring from unfertilised eggs, sexual traits are expected to decay (van der Kooi and Schwander 2014). Both male and female sexual traits are subject to decay. However, the decay of male sexual traits has only been documented for a limited number of asexual species, compared to female sexual traits (van der Kooi and Schwander 2014). For example, males of three endoparasitoid wasp species of the Encarsia genus showed degradation of courtship and mating behaviours, although the sperm production was still normal (Zchori-Fein et al. 1992; Giorgini 2001). Evidence for sperm abnormalities in asexually produced males was found in the parasitoid wasp Muscidifurax uniraptor (Gottlieb and Zchori-Fein 2001) and the snail Potamopyrgus antipodarum (Jalinsky et al. 2020).
Interestingly, several female traits such as the ones involved in mate location and copulation behaviours have been shown to decay faster compared to male sexual traits (Heethoff et al. 2007; van der Kooi and Schwander 2014). A difference in rate of decay has been suggested to depend on whether these traits are neutral (non-functional) or maladaptive (Heethoff et al. 2007; van der Kooi and Schwander 2014). For traits that are neutral within the new selective environment, the decay should result from accumulation of loss-of-function mutations, a process that is expected to be stochastic and slow (Muller 1949; Kimura 1983; Hall and Colegrave 2008). On the contrary, a formerly adaptive trait that is costly to maintain due to environmental selection (i.e. predation) and/or to high energy investment, is expected to deteriorate faster as a consequence of negative selection against that trait (Schwander et al. 2013; Hall and Colegrave 2008).
Female reproductive traits were found to decay faster and more consistently in the majority of the measured asexual lineages (van der Kooi and Schwander 2014), with independent transitions to asexuality showing parallel decay of these traits (Schwander et al. 2013). Conversely, no evidence of parallel male trait decay was found across asexual lineages. This suggests that sexual traits of males decay more slowly, as a consequence of drift rather than selective processes (van der Kooi and Schwander 2014).
In the springtail Folsomia candida Willem, both sexual and asexual lineages have been described (Fountain and Hopkin 2005; Tully et al. 2006; Krogh et al. 2008; Tully and Potapov 2015; Goto 1960; Frati et al. 2004; Buono and Tully 2011). Asexual lines carry the obligate intracellular bacterium Wolbachia, which plays a crucial role in the successful parthenogenetic development of females, as antibiotic treatment inhibits egg development in F. candida (Timmermans and Ellers 2009; Pike and Kingcombe 2009). In line with what is observed in a variety of animal taxa, asexual females occasionally produce males (van der Kooi and Schwander 2014). F. candida is a diplodiploid species (XO:XX), with males being the heterogametic sex. Although the exact cellular mechanism underling the rare male development is not confirmed, it can be hypothesized that an error occurs during the fusion of the haploid egg with the polar body or during the post-zygotic development, leading to the elimination of an X chromosome. Males are produced at a very low rate by asexual F. candida, with only one male out of 1000 to 10,000 individuals (Krogh et al. 2008; Buono and Tully 2011). These rare males seem to be sterile, as keeping them in an equal ratio together with females does not lead to shared offspring, nor are they able to fertilise the eggs of sexual populations (Krogh et al. 2008; Buono and Tully 2011). Thus, male trait decay is expected to have occurred in asexual F. candida. However, which sexual traits are degraded and to what extent has yet to be tested. In this study, we investigated male sexual traits in one sexual and two parthenogenetic populations of F. candida.
Although Folsomia candida is a widely established model organism, the observations reported so far have yielded little information regarding the reproductive behaviour of the sexual strains. Evidence suggests that sexual individuals of F. candida have an equal sex ratio (Goto 1960; Buono and Tully 2011). An indirect transfer of sperm via stalked sperm packages (from now referred to as spermatophores) deposited on the soil has been suggested. However, it is to be confirmed whether the mating behaviour of sexual individuals is in line with what is reported in other collembolans. Our study provides the first detailed description of the reproductive behaviour of sexual F. candida.
A common mode of reproduction in soil arthropods, consists of females picking up a spermatophore for internal fertilisation without any physical contact with the male (Schaller 1971; Proctor 1998; Zizzari et al. 2017). In such a mating system, referred to as dissociated sperm transfer (Alexander 1964), spermatophore-related traits play a crucial role in male reproductive success, as the spermatophores convey all the information used by females to assess the quality of their ‘mate’ (Zizzari et al. 2009, 2013, 2017). Therefore, in this study we analysed several spermatophore-related traits: stalk length, sperm droplet size, amount of sperm DNA material and attractiveness. Specifically, we tested whether these traits are subject to decay and whether a parallel trait regression occurred in both asexual F. candida lines.
Methods
Folsomia candida Cultures
One sexual and two parthenogenetic lines of F. candida were used. The sexual population (from now on “CR”) was collected in Cremona (Italy) and described by Frati et al. (2004). One of the parthenogenetic lines used was the “Berlin strain” (from now on “BE”) which is commonly used for lab tests (Vrije Universiteit, Amsterdam). The other parthenogenetic line used in this study was collected in a ground water sample in October 2013 in Evergem (from now on “EV”), Belgium, and kept in the lab of Vrije Universiteit Amsterdam since. All cultures were kept in pots with a water-saturated plaster of Paris bottom containing 10% charcoal at 20 °C in a 16:8 light dark regime and were fed dried baker’s yeast (Dr. Oetker) ad libitum.
Reproductive Biology of Sexual F. candida
The reproductive biology of several collembolan species has been described in detail. Upon maturation males and females alternate reproductive and non-reproductive instars, separated by moults (see e.g., Poggendorf 1956; Waldorf 1971, 1975; Zizzari et al. 2017). Males deposit the spermatophores only during reproductive instars, while females pick up a single spermatophore at the onset of each reproductive instar to fertilise all the eggs, as they do not store sperm (Waldorf 1971; Gols et al. 2004; Dallai et al. 2008). To characterize the reproductive biology of the sexual CR population, collembolans were kept individually in plexiglas vials (2.4 cm) with a bottom of water-saturated plaster of Paris containing 10% charcoal and a lid with fine meshed gauze at culture conditions. To measure the juvenile instar length (in days) and determine age of first reproduction, hatchlings were isolated (N = 20) and checked daily for the presence of moulting skins. Females will only start laying eggs after taking up a spermatophore, therefore, nearly adult females were offered freshly laid spermatophores soon after moulting and were subsequently checked for egg laying, following a protocol established for other collembolans (Zizzari et al. 2009, 2013, 2017). As for the males, we monitored each individual for the presence of spermatophores in the vial. After reaching reproductive maturity, the number of spermatophores produced daily (males, N = 30) and the number of eggs (females, N = 20) were scored during their first reproductive instar. For first egg clutches (N = 20), clutch size, hatching time and sex ratio were also determined. During the characterisation of the reproductive biology we noticed that male reproductive instars lasted longer than non-reproductive instars, thus we tested if this difference was significant by comparing the lengths of the first reproductive instars with the lengths of the subsequent non-reproductive instar. Finally, we tested if female reproductive instar length would change when no spermatophore was offered by comparing instar lengths of females in the 10th instar with and without spermatophores present (N = 24).
Male Sexual Traits
Individually kept males of the three lineages (CR, N = 32; BE, N = 23; EV, N = 9) were daily monitored for the presence of spermatophores to be used for the measurements of stalk length, sperm droplet size, amount of sperm DNA material, and for conducting the female choice trials.
Morphological Characteristics of the Spermatophore
Spermatophores of the sexual (N = 21) and asexual lines (EV, N = 9; BE, N = 12) were collected and mounted on microscope slides to measure stalk length, sperm droplet size, and amount of sperm DNA material in the sperm droplets. Each spermatophore was mounted on a microscope slide and stained with a DAPI solution (VECTASHIELD Mounting Medium with 4′,6-diamidino-2-phenylindole 1.5 µg/ml), following Dallai et al. (2009). DAPI binds to the DNA and as the spermatophore coat (i.e. the external layer) of Collembola does not contain cell nuclei or DNA (Dallai et al. 2009), blue coloration indicates the presence of sperm DNA material only. Pictures of the stained spermatophores were taken with a camera (ZEISS AxioCam ICc 3) mounted on a fluorescence microscope (ZEISS Axioskop 20) with a × 1000 magnification, and the area of DAPI stained sperm droplet was measured (ImageJ, version 1.46).
Spermatophore Attractiveness
To evaluate the attractiveness of spermatophores to females, we conducted two series of two-choice trials using a Y-shaped glass olfactometer with vials attached to the proximal end of the choice arms (Fig. S1). First, we tested the ability of females of the sexual CR population to detect the presence of spermatophores. For this test, virgin receptive females of the sexual population (N = 36) were offered a choice between a vial with spermatophores and an empty vial. Subsequently, we assessed whether sexual females were attracted to spermatophore of the asexual lines over empty vials (BE, N = 39; EV, N = 26). In the second series of two-choice trials, we tested weather females could differentiate between vials containing spermatophores of different strains: sexual CR vs. asexual BE (N = 42), sexual CR vs. asexual EV (N = 35), and asexual BE vs. asexual EV (N = 23). Vials with fresh spermatophores were obtained by placing males in individual vials one day before the start of the female choice trials. Before each trial, the vials containing spermatophores were mounted at the extremities of the choice arms of the olfactometers. Choices were scored when a female walked into one of the arms for more than 5 mm. Choice tests were terminated after 10 min, regardless of whether the female had made a choice. After the test, females were placed in a vial with spermatophores of a sexual male to check if they were receptive during the test.
Statistics
Lengths of reproductive and non-reproductive instars of the sexual CR population were compared using Wilcoxon rank sum tests with continuity correction. All three lineages of F. candida were tested for differences in stalk length, sperm droplet size and amount of sperm cell within the sperm droplet. Assumptions of normally distributed residuals and homogenous variance between the different treatments were tested with the Shapiro–Wilk normality test and Bartlett test of homogeneity of variances, respectively. Stalk length met both criteria and was therefore analysed with a one-way ANOVA. Sperm droplet size and amount of sperm did not meet the requirements of normality. Therefore, these traits were analysed using a non-parametrical Kruskal–Wallis rank sum test. Wilcoxon–Mann–Whitney tests were employed to assess pairwise differences between lineages, as posthoc comparisons. To test female preference in the behavioural assays we applied a Chi-squared test against a H0 of no choice. Females that did not enter one of the arms within the observation time were excluded from statistical analysis. All tests were run in R (R Core Team 2017).
Results
Reproductive Biology of Sexual F. candida
In order to characterize the reproductive traits of the sexual CR population of F. candida we monitored individual springtails daily for ten weeks (Fig. 1). As expected, both males and females of the sexual population alternated reproductive with non-reproductive instars. Females took up a fresh spermatophore each reproductive instar and laid on average 31 (± 12) eggs in their first reproductive instar. The development time of the eggs was 10.1 (± 0.3) days. The sex ratio of the sexual clutches was 48.7% males to 51.3% females, which does not deviate significantly from an equal distribution (binomial-test, R, P = 0.48). Both the number of days as well as the number of instars until first reproduction differed between both sexes. Female age at maturity was 17.2 (± 1.6) days in the 6th instar, whereas males developed faster and reached the first reproductive instar after 14.9 (± 1.4) days in the 5th instar. The first male reproductive instar, with on average 172 (± 95) spermatophores, was significantly longer than the subsequent non-reproductive instar (W = 465, P < 0.001). An increased number of spermatophores laid during the last days of the first reproductive instar was also recorded (Fig. S2). In addition, female reproductive instars were longer when no spermatophores were available compared to when spermatophores were available (W = 470.5, P < 0.001).
Reproductive biology of the sexual CR population of F. candida at 20 °C. Individuals keep growing through successive moults throughout their lifespan. For both males and females, the instars (intermoult periods) are numbered. After maturity, males and females alternate reproductive and non-reproductive instars. Juvenile instars are depicted in grey. Males produce spermatophores throughout the whole reproductive instar and females pick up a new spermatophore at the start of each reproductive instar to fertilise their eggs. Time intervals are given in days ± standard deviation (SD). Number of spermatophores and number of eggs during the first reproductive instar are given in number ± SD. Male and female ratios are given as a percentage
Male Sexual Traits
Morphological Characteristics of the Spermatophore
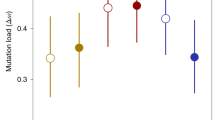
To detect degradation patterns in male sexual traits, we measured stalk length, sperm droplet size and amount of sperm of male spermatophores in the three different populations. The stalk length did not differ significantly among the three lineages (F(2,39) = 2.778, P = 0.075; Fig. 2a). The sperm droplet size varied significantly (χ2(2) = 9.0464, P = 0.011; Fig. 2b), with the asexual EV line having larger sperm droplets than the asexual BE line (z = − 2.9147, P = 0.0036) and the sexual population (z = 2.512, P = 0.012). No differences were found between the sexual population and the asexual BE line (z = − 0.4678, P = 0.639). The amount of sperm with DNA differed significantly among all three lineages (χ2(2) = 29.371, P < 0.001; Fig. 2c). The sexual population showed significantly more fluorescent sperm heads compared to both the asexual lines (EV: z = − 2.8738, P = 0.004; BE: z = − 4.7158, P < 0.001; Fig. 2d). The sperm droplets of the asexual BE line contained significantly fewer DNA material than the asexual EV line (z = − 3.8389, P < 0.001; Fig. 2d).
Spermatophore-related traits of three F. candida lines: stalk length (a), sperm droplet size (b), amount of sperm heads measured as DAPI stained area (c) and fluorescence microscope view of DAPI-stained spermatophores (d). Box plots depict the first to third quartiles with the median as inner line and whiskers showing the maximum and minimum, except for when outliers are present, in which case they show the inner fences
Spermatophore Attractiveness
In a series of two-choice tests we tested spermatophore attractiveness to receptive females of the sexual line CR. CR females were significantly more attracted to spermatophores of males of their own population than to empty vials (χ2(1) = 16.03, P < 0.001; Fig. 3). They also preferred spermatophores of the asexual BE line over empty vials (χ2(1) = 8.76, P < 0.01; Fig. 3) but did not show any preference for spermatophores of the asexual EV line (χ2(1) = 1.09, P = 0.297; Fig. 3). In a second two-choice trial, we tested whether sexual CR females differentiated between spermatophores of the different lines. Spermatophores of the sexual CR population were significantly more attractive to females than the asexual EV spermatophores (χ2(1) = 4.5, P < 0.05; Fig. 3), but CR females showed no significant preference for the CR spermatophores compared to the spermatophores of the asexual BE line (χ2(1) = 0.64, P = 0.423; Fig. 3). Also, no significant preference was detected between spermatophores of the two asexual lines (χ2(1) = 0.47, P = 0.491; Fig. 3).
Behavioural responses of sexual F. candida females to spermatophores of different F. candida lines in a Y-olfactometer. Origin of spermatophores in the two-choice tests is shown on the right. Numbers in brackets indicate the absolute number of females that chose. Total number of tested females is given in the text. Asterisks indicate significant differences determined by a Chi-squared test for given probabilities (* is P < 0.05, ** is P < 0.01, *** is P < 0.001). Inside bar patterns indicate the average amount of sperm for each lineage: sexual CR > asexual EV > asexual BE
Discussion
In this study, we investigated male sexual trait decay in the asexual springtail F. candida by comparing spermatophore-related traits of two asexual lines and one sexual population, whose reproductive biology is described here for the first time. In the asexual lines, we found evidence for decay of the amount of sperm DNA material within the sperm droplet and of spermatophore attractiveness. Moreover, our results suggest that the extent of decay of such male sexual traits differs between the two asexual lineages.
Reproductive Biology of Sexual F. candida
While the reproductive traits of asexual F. candida are well-documented (Fountain and Hopkin 2005; Krogh et al. 2008; Snider and Butcher 2017), those of sexual populations have not received the same attention. Our study provides useful information on the mode of sperm transfer and on the reproductive behaviour of sexual F. candida. We showed that sexual males deposit spermatophores in the absence of females, thus confirming a dissociated sperm transfer system. Males reach maturity on average two days before females and the reproductive instar is longer than the non-reproductive instar. Faster maturation of males is common in Collembola and other invertebrates (Poggendorf 1956; Waldorf 1971, 1975; Zizzari et al. 2016). A longer male reproductive instar with an increase in spermatophore deposition toward its end, as found in F. candida, has also been observed in other Collembola (Testerink 1982). Prolonging the reproductive instars could be a way to maximize male reproductive success, as it gives a male a longer time window in which spermatophore deposition can occur (Waldorf 1971). Females appear to have plastic reproductive instar lengths, as we found the reproductive instar to be prolonged in the absence of spermatophores. In two-choice trials, receptive females showed a strong preference for vials containing spermatophores over empty vials. These findings confirm that also females of F. candida locate spermatophores based on chemical cues and add to the increasing evidence that collembolan spermatophore-associated pheromones mediate partner attraction (Zizzari et al. 2017). Our study shows that egg development time, age at first reproduction, and number of juvenile instars were within the range observed in females of asexual lines. However, the average clutch size of sexual females was lower than observed in asexual females (Fountain and Hopkin 2005; Krogh et al. 2008; Snider and Butcher 2017).
In conclusion, a continuous moulting throughout the life span, the alternation of reproductive and non-reproductive instars, and female attraction to spermatophore odour, confirm that the reproductive biology of sexual F. candida is in line with what is observed in other collembolans species. Overall, this life-history information makes an important laboratory model available for further investigation on the transition from sexual to asexual reproduction promoted by Wolbachia and on the evolutionary dynamics of sexual traits loss.
Decay of Male Traits in Asexual F. candida
Spermatophore-Related Traits
We found a strong indication that in asexual F. candida, sperm production is subject to decay. Both asexual lines had a lower amount of sperm DNA material than the sexual population and in the spermatophores of one of the asexual lines, fluorescent sperm heads were even almost completely absent. Our results are in line with previous findings of sperm production to be one of the first male traits to deteriorate in asexual lines, which has been reported in several species (reviewed in van der Kooi and Schwander 2014).
In addition to a reduction in sperm production, spermatophore scent was another male sexual trait observed to be degraded. We evaluated the olfactory attractiveness of spermatophores to sexual females in two-choice trials. Spermatophores of the sexual line were significantly more attractive than the asexual EV spermatophores, while females showed no preference for sexual spermatophores over asexual BE spermatophores. The observed reduced attractiveness for asexual EV spermatophores may be due to decay of the pheromone biosynthetic pathway, leading to a reduced or modified sex pheromone expression. A variation of the pheromone profile, owing to the absence of directional selection, could also explain the reduced spermatophore preference, as fine-tuning of sexual signals and reception can only be maintained under strong selection (Lindstrom and Kotiaho 2002; Groot and Zizzari 2019). Given that these signals experience weak selection in asexuals, alteration of the expression of formerly adaptive olfactory cues can be expected. Alternatively, population-specific mating preferences, as shown for instance in the parasitoid wasp Leptopilina clavipes (Kraaijeveld et al. 2009), could be hypothesized. Thus, a difference in spermatophore attractiveness may have been due to differences in sex pheromone profile among populations. The characterization of the pheromone bouquets emitted by the spermatophores of the three investigated populations would help elucidating the observed patterns in spermatophore preference.
A shift in the sperm droplet size was detected in only one of the asexual lines, with an increase in size rather than the typical decrease associated with trait decay. Thus, the sperm droplets of males of the asexual line EV were larger than those of the other two examined lineages. However, the size of sperm and spermatophores often co-evolves with width and size of the female reproductive tract (see e.g., Presgraves et al. 1999; Dallai et al. 2014). For this reason, a divergence in the size of a spermatophore, either larger or smaller, is likely an indication of weak co-evolutionary constraints that determine the optimal size of the spermatophore to be picked up by a female.
We tentatively suggest that male sexual traits have not decayed in parallel in the two asexual lines. Although the difference in spermatophore attractiveness could be a consequence of lineage diversification, this is not the case for variation in the amount of sperm DNA material in the sperm droplets. Such a key trait is unlikely to be under divergent selection between populations and therefore presents strong evidence for sexual trait decay in the males.
Assuming the differences in spermatophore attractiveness, sperm droplet size, and amount of sperm are indeed a consequence of decay, the question arises as to which evolutionary forces promote the different rates of decay. Mutation accumulation is the result of a stochastic process (Kimura 1983); hence, progression of trait decay is expected to differ between asexual lines. Conversely, if a sexual trait is subject to strong regression in old asexual lines as well as in young ones, as was shown in asexual female Timema (Schwander et al. 2013), the causative evolutionary process is more likely negative selection. Although we do not know the number of independent transitions of the asexual lines, the pattern of trait decay found in our study appears to be consistent with neutral mutation accumulation rather than selection.
Since trait decay by neutral mutation accumulation can be found in long-lived asexual lineages only (Kimura 1983; Hall and Colegrave 2008; van der Kooi and Schwander 2014), we suggest Folsomia to be an ancient asexual. Male trait decay was detected in oribatid mites which are estimated to having been parthenogenetic for 100 million years (Heethoff et al. 2007; Martens et al. 2003). The high genetic differentiation between sexuals and two Italian asexual lines (Frati et al. 2004), might support ancient asexuality in Folsomia, although genetic variation can also be associated with ecological niche differentiation, as shown in asexual grass thrips (van der Kooi et al. 2019).
Future studies should shed light upon the intraspecific molecular divergence between the Folsomia sexual and asexual lineages BE and EV to estimate for how long they have been parthenogenetic. Moreover, further investigation should ascertain whether spermatophores of asexually produced males contain viable sperm and whether Wolbachia-infected females can produce offspring with spermatophores of sexual males.
Data Availability
All data generated or analysed during the current study are included in this published article and its Supplementary Information file.
References
Alexander, R. D. (1964). The evolution of mating behavior in arthropods. In K. C. Highnam (Ed.), Insect reproduction (pp. 78–94). London: Royal Entomological Society.
Buono, L., & Tully, T. (2011). Etude préliminaire des modes de reproduction chez Folsomia candida. Master 1 EBE, Université Pierre et Marie Curie.
Dallai, R., Gottardo, M., Mercati, D., Machida, R., Mashimo, Y., Matsumura, Y., & Beutel, R. G. (2014). Giant spermatozoa and a huge spermatheca: A case of coevolution of male and female reproductive organs in the ground louse Zorotypus impolitus (Insecta, Zoraptera). Arthropod Structure and Development, 43, 135–151.
Dallai, R., Zizzari, Z. V., & Fanciulli, P. P. (2008). Fine structure of the spermatheca and of the accessory glands in Orchesella villosa (Collembola, Hexapoda). Journal of Morphology, 269, 464–478.
Dallai, R., Zizzari, Z. V., & Fanciulli, P. P. (2009). Different sperm number in the spermatophores of Orchesella villosa (Geoffroy) (Entomobryidae) and Allacma fusca (L.) (Sminthuridae). Arthropod Structure and Development, 38, 227–234.
Ellers, J., Toby Kiers, E., Currie, C. R., Mcdonald, B. R., & Visser, B. (2012). Ecological interactions drive evolutionary loss of traits. Ecology Letters, 15, 1071–1082.
Fong, D. W., Kane, T. C., & Culver, D. C. (1995). Vestigialization and causes of vestigialization. The Annual Review of Ecology and the Systematics, 26, 249–268.
Fountain, M. T., & Hopkin, S. P. (2005). Folsomia candida (Collembola): A “standard” soil arthropod. Annual Review of Entomology, 50, 201–222.
Frati, F., Negri, I., Fanciulli, P. P., Pellecchia, M., De Paola, V., Scali, V., & Dallai, R. (2004). High levels of genetic differentiation between Wolbachia-infected and non-infected populations of Folsomia candida (Collembola, Isotomidae). Pedobiologia, 48, 461–468.
Giorgini, M. (2001). Induction of males in thelytokous populations of Encarsia meritoria and Encarsia protransvena: A systematic tool. BioControl, 46, 427–438.
Gols, R., Ernsting, G., & Van Straalen, N. M. (2004). Paternity analysis in a hexapod (Orchesella cincta; Collembola) with indirect sperm transfer. Journal of Insect Behavior, 17, 317–328.
Goto, H. E. (1960). Facultative parthenogenesis in Collembola (Insecta). Nature, 188, 958–959.
Gottlieb, Y., & Zchori-Fein, E. (2001). Irreversible thelytokous reproduction in Muscidifurax uniraptor. Entomologia Experimentalis et Applicata, 100, 271–278.
Groot, A. T., & Zizzari, Z. V. (2019). Does climate warming influence sexual chemical signaling? Animal Biology, 69, 83–93.
Hall, A. R., & Colegrave, N. (2008). Decay of unused characters by selection and drift. Journal of Evolutionary Biology, 21, 610–617.
Heethoff, M., Domes, K., Laumann, M., Maraun, M., Norton, R. A., & Scheu, S. (2007). High genetic divergences indicate ancient separation of parthenogenetic lineages of the oribatid mite Platynothrus peltifer (Acari, Oribatida). Journal of Evolutionary Biology, 20, 392–402.
Jalinsky, J., Logsdon, J. M., Jr., & Neiman, M. (2020). Male phenotypes in a female framework: Evidence for degeneration in sperm produced by male snails from asexual lineages. Journal of Evolutionary Biology. https://doi.org/10.1111/jeb.13632
Kimura, M. (1983). The neutral theory of molecular evolution. Cambridge: Cambridge University Press.
Kraaijeveld, K., Franco, P., Reumer, B. M., & van Alphen, J. J. M. (2009). Effects of parthenogenesis and geographic isolation on female sexual traits in a parasitoid wasp. Evolution, 63, 3085–3096.
Krogh, P. H., João, M., Amorim, D. B., Andrés, P., Van Slooten, K. B., Domene, X., et al. (2008). Toxicity testing with the collembolans Folsomia fimetaria and Folsomia candida and the results of a ringtest. Ecology, 23, 1–44.
Lahti, D. C., Johnson, N. A., Ajie, B. C., Otto, S. P., Hendry, A. P., Blumstein, D. T., Coss, R. G., Donohue, K., & Foster, S. A. (2009). Relaxed selection in the wild. Trends in Ecology & Evolution, 24, 487–496.
Lindstrom, L., & Kotiaho, J. S. (2002). Signalling and reception. Encyclopedia of life sciences. New York: Macmillan.
Martens, K., Rossetti, G., & Horne, D. J. (2003). How ancient are ancient asexuals? Proceedings of the Royal Society of London. Series B, 270, 723–729.
Muller, H. J. (1949). The Darwinian and modern conceptions of natural selection. Proceedings of the American Philosophical Society, 93, 459–470.
Pike, N., & Kingcombe, R. (2009). Antibiotic treatment leads to the elimination of Wolbachia endosymbionts and sterility in the diplodiploid collembolan Folsomia candida. BMC Biology, 7, 54.
Poggendorf, D. (1956). Über rhythmische sexuelle Aktivität und ihre Beziehungen zu Häutung und Haarbildung bei arthropleonen Collembolen (Springschwanze). Naturwissenschaften, 43, 45–45.
Presgraves, D. C., Baker, R. H., & Wilkinson, G. S. (1999). Coevolution of sperm and female reproductive tract morphology in stalk-eyed flies. Proceedings of the Royal Society B: Biological Sciences, 266, 1041–1047.
Proctor, H. C. (1998). Indirect sperm transfer in asthropods: Behavioral and evolutionary trends. Annual Review of Entomology, 43, 153–174.
R Core Team. (2017). R: A language and environment for statistical computing. Vienna: R Development Core Team.
Schaller, F. (1971). Indirect sperm transfer. Annual Review of Entomology, 16, 407–446.
Schwander, T., Crespi, B. J., Gries, R., & Gries, G. (2013). Neutral and selection-driven decay of sexual traits in asexual stick insects. Proceedings of the Royal Society B: Biological Sciences, 2801764, 20130823.
Snider, R. M., & Butcher, J. W. (2017). The life history of Folsomia candida (Willem) (Collembola: Isotomidae) relative to temperature. The Great Lakes Entomologist, 6, 97–106.
Testerink, G. J. (1982). Strategies in energy consumption and partitioning in Collembola. Ecological Entomology, 7, 341–351.
Timmermans, M. J. T. N., & Ellers, J. (2009). Wolbachia endosymbiont is essential for egg hatching in a parthenogenetic arthropod. Evololutionary Ecology, 23, 931–942.
Tully, T., D’Haese, C. A., Richard, M., & Ferrière, R. (2006). Two major evolutionary lineages revealed by molecular phylogeny in the parthenogenetic collembola species Folsomia candida. Pedobiologia (Jena), 50, 95–104.
Tully, T., & Potapov, M. (2015). Intraspecific phenotypic variation and morphological divergence of strains of Folsomia candida (Willem) (Collembola: Isotomidae), the “Standard” test springtaill. PLoS ONE, 10, e0136047.
van der Kooi, C. J., Ghali, K., Amptmeijer, D., & Schwander, T. (2019). Niche differentiation among clones in asexual grass thrips. Journal of Evolutionary Biology, 32, 126–130.
van der Kooi, C. J., & Schwander, T. (2014). On the fate of sexual traits under asexuality. Biological Reviews, 89, 805–819.
Waldorf, E. (1975). Grooming through the reproductive cycle in male Sinella Coeca (Collembola: Entomobryidae). Psyche (New York), 82, 359–365.
Waldorf, E. S. (1971). The reproductive biology of Sinella curviseta (Collembola: Entomobryidae) in laboratory culture. Revue d’écologie et de biologie du sol, 8, 451–463.
Zchori-Fein, E., Roush, R. T., & Hunter, M. S. (1992). Male production induced by antibiotic treatment in Encarsia formosa (Hymenoptera: Aphelinidae), an asexual species. Experientia, 48, 102–105.
Zizzari, Z. V., Braakhuis, A., van Straalen, N. M., & Ellers, J. (2009). Female preference and fitness benefits of mate choice in a species with dissociated sperm transfer. Animal Behaviour, 78, 1261–1267.
Zizzari, Z. V., Engl, T., Lorenz, S., van Straalen, N. M., Ellers, J., & Groot, A. T. (2017). Love at first sniff: A spermatophore-associated pheromone mediates partner attraction in a collembolan species. Animal Behaviour, 124, 221–227.
Zizzari, Z. V., van Straalen, N. M., & Ellers, J. (2013). Male-male competition leads to less abundant but more attractive sperm. Biology Letters, 9, 20130762.
Zizzari, Z. V., van Straalen, N. M., & Ellers, J. (2016). Transgenerational effects of nutrition are different for sons and daughters. Journal of Evolutionary Biology, 29, 1317–1327.
Acknowledgements
We thank Janine Mariën and Rudo A. Verweij for maintaining the cultures of asexual F. candida lines and Prof. P. Paolo Fanciulli for providing us with the sexual population of F.candida. Finally, we are grateful to the anonymous reviewers for insightful comments.
Funding
Open Access funding provided by Projekt DEAL.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflicts of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Figure S1
The response of F. candida female to different odour source was tested in a glass Y-tube olfactometer (1 cm inner diameter) with an introduction arm (6 cm) and two choice arms (6 cm). The angle between the two choice arms was 50. Individual females were placed into the introduction chamber from which they locomoted to the Y-intersection and then selected a Y-arm to enter. Two scent sources (i.e., Plexiglas vials) were attached to the proximal end of the choice arms. A fine mesh dived the vials from the olfactometer. The set-up could be disassembled for cleaning between trials. (PNG 513 kb)
Figure S2
Male spermatophore production in F. candida sexual line during the first reproductive instar. The figure shows the percentage of spermatophores laid (± SE), for each day prior to moult. The numbers of males that could be included per day is shown above each bar. (EMF 236 kb)
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Kampfraath, A.A., Dudink, T.P., Kraaijeveld, K. et al. Male Sexual Trait Decay in Two Asexual Springtail Populations Follows Neutral Mutation Accumulation Theory. Evol Biol 47, 285–292 (2020). https://doi.org/10.1007/s11692-020-09511-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11692-020-09511-z