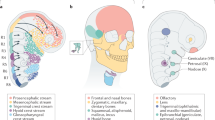
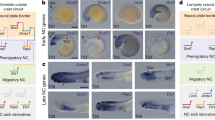
Abstract
I evaluate the lines of evidence—cell types, genes, gene pathways, fossils—in putative chordate ancestors—cephalochordates and ascidians—pertaining to the evolutionary origin of the vertebrate neural crest. Given the intimate relationship between the neural crest and the dorsal nervous system during development, I discuss the dorsal nervous system in living (extant) members of the two groups, especially the nature, and genes, and gene regulatory networks of the brain to determine whether any cellular and/or molecular precursors (latent homologues) of the neural may have been present in ancestral cephalochordates or urochordates. I then examine those fossils that have been interpreted as basal chordates or cephalochordates to determine whether they shed any light on the origins of neural crest cell (NCC) derivatives. Do they have, for example, elements of a head skeleton or pharyngeal arches, two fundamental vertebrate characters (synapomorphies)? The third topic recognizes that the origin of the neural crest in the first vertebrates accompanied the evolution of a brain, a muscular pharynx, and paired sensory organs. In a paradigm-breaking hypothesis—often known as the ‘new head hypothesis’—Carl Gans and Glen Northcutt linked these evolutionary innovations to the evolution of the neural crest and ectodermal placodes (Gans and Northcutt Science 220:268-274, 1983. doi:10.1126/science.220.4594.268; Northcutt and Gans The Quarterly Review of Biology 58:1–28, 1983. doi:10.1086/413055). I outline the rationale behind the new head hypothesis before turning to an examination of the pivotal role played by NCCs in the evolution of pharyngeal arches, in the context of the craniofacial skeleton. Integrations between the evolving vertebrate brain, muscular pharynx and paired sensory organs may have necessitated that the pharyngeal arch skeletal system—and subsequently, the skeleton of the jaws and much of the skull (the first vertebrates being jawless)—evolved from NCCs whose developmental connections were to neural ectoderm and neurons rather than to mesoderm and connective tissue; mesoderm produces much of the vertebrate skeleton, including virtually all the skeleton outside the head. The origination of the pharyngeal arch skeleton raises the issue of the group of organisms in which and how cartilage arose as a skeletal tissue. Did cartilage arise in the basal proto-vertebrate from a single germ layer, cell layer or tissue, or were cells and/or genes co-opted from several layers or tissues? Two recent studies utilizing comparative genomics, bioinformatics, molecular fingerprinting, genetic labeling/cell selection, and GeneChip Microarray technologies are introduced as powerful ways to approach the questions that are central to this review.




Similar content being viewed by others
Notes
As this review deals with the origination rather than the evolution of diversification and specialization of neural crest cells, I do not discuss the evidence for neural crest cells and their derivatives in lampreys and hagfishes, nor do I discuss the phylogenetic relationships between lampreys and hagfishes. For entrees into the literatiure on these topics, see Kuratani et al. (2002), Meulemans and Bronner-Fraser (2002), Janvier (2007), Ota and Kuratani 2006, 2007, and Ota et al. (2007).
For many years, Col2a1 has been regarded as the “cartilage collagen gene”. However, we will see that Col2a1 is expressed in the notochord and in endodermal cells. Furthermore, many NCCs express Col2a1, often in combination with genes such as Sox9, Sox10, and LSox5, which activate the collagen gene. So general is this combination of gene expressions that Suzuki et al. (2006)) concluded that Col2a1 is a “general mesenchyme gene.”.
References
Baker, C. V. H., & Bronner-Fraser, M. (1997). The origins of the neural crest. Part II: An evolutionary perspective. Mechanisms of Development, 69, 13–29. doi:10.1016/S0925-4773(97)00129-9.
Basch, M. L., Bronner-Fraser, M., & Garcia-Castro, M. I. (2006). Specification of the neural crest occurs during gastrulation requires Pax7. Nature, 441, 218–222. doi:10.1038/nature04684.
Bhattacherjee, V., Mukhopadhyay, P., Singh, S., Johnson, C., et al. (2007). Neural crest and mesoderm lineage-dependent gene expression in orofacial development. Differentiation, 75, 463–477. doi:10.1111/j.1432-0436.2006.00145.x.
Bourlat, S. J., Juliusdottir, T., Lowe, C. J., Freeman, R., et al. (2006). Deuterostome phylogeny reveals monophyletic chordates and the new phylum Xenoturbellida. Nature, 444, 85–88. doi:10.1038/nature05241.
Cañestroa, C., & Postlethwait, J. H. (2007). Development of a chordate anterior-posterior axis without classical retinoic acid signaling. Developmental Biology, 305, 522–538. doi:10.1016/j.ydbio.2007.02.032.
Chen, J.-Y., Huang, D.-Y., & Li, C.-W. (1999). An early Cambrian craniate-like chordate. Nature, 402, 518–522. doi:10.1038/990080.
Cohn, M. J. (2002). Lamprey Hox genes and the origin of jaws. Nature, 416, 386–387. doi:10.1038/416386a.
Cole, A. G., & Hall, B. K. (2004a). Cartilage is a metazoan tissue; integrating data from non-vertebrate sources. Acta Zoologica (Stockholm), 85, 69–80.
Cole, A. G., & Hall, B. K. (2004b). The nature and significance of invertebrate cartilages revisited: Distribution and histology of cartilage and cartilage-like tissues within the Metazoa. Zoology (Jena, Germany), 107, 261–274. doi:10.1016/j.zool.2004.05.001.
Cole, A. G., & Hall, B. K. (2008). Cartilage differentiation in cephalopod molluscs. Zoology (Jena, Germany), in press.
Corbo, J. C., Erives, A., DiGregorio, A., Chang, A., & Levine, M. (1997). Dorsoventral patterning of the vertebrate neural tube is conserved in a protochordate. Development, 124, 2335–2344.
Dehal, P., Satou, Y., Campbell, R. K., Chapman, J., et al. (2002). The draft genome of Ciona intestinalis: Insights into chordate and vertebrate origins. Science, 298, 2157–2167. doi:10.1126/science.1080049.
Delsuc, F., Brinkmann, H., Chourrout, D., & Philippe, H. (2006). Tunicates and not cephalochordates are the closest living relatives of vertebrates. Nature, 439, 965–968. doi:10.1038/nature04336.
Donoghue, P. C. J. (2002). Evolution of development of the vertebrate dermal and oral skeletons: Unraveling concepts, regulatory theories, and homologies. Paleobiology, 28, 474–507. doi :10.1666/0094-8373(2002)028<0474:EODOTV>2.0.CO;2.
Dufour, H. D., Chettouh, Z., Deyts, C., De Rosa, R., et al. (2006). Precraniate origin of cranial motoneurons. Proceedings of the National Academy of Sciences of the United States of America, 103, 8727–8732. doi:10.1073/pnas.0600805103.
Ellies, D. L., Langille, R. M., Martin, C. C., Akimenko, M.-A., & Ekker, M. (1997). Specific craniofacial cartilage dysmorphogenesis coincides with a loss of Dlx gene expression in retinoic acid-treated zebrafish embryos. Mechanisms of Development, 61, 23–36. doi:10.1016/S0925-4773(96)00616-8.
Ericsson, R., Cerni, R., Falck, P., & Olsson, L. (2004). Role of cranial neural crest cells in visceral arch muscle positioning and morphogenesis in the Mexican axolotl, Ambystoma mexicanum. Developmental Dynamics, 231, 237–247. doi:10.1002/dvdy.20127.
Escriva, H., Holland, N. D., Gronemeyer, H., Laudet, V., & Holland, L. Z. (2002). The retinoic acid signaling pathway regulates anterior/posterior patterning in the nerve cord and pharynx of amphioxus, a chordate lacking neural crest. Development, 129, 2905–2916.
Fritzsch, B., & Northcutt, R. G. (1993). Cranial and spinal nerve organization in amphioxus and lampreys: Evidence for an ancestral craniate pattern. Acta Anatomica, 148, 96–109. doi:10.1159/000147529.
Gans, C., & Northcutt, R. G. (1983). Neural crest and the origin of vertebrates: A new head. Science, 220, 268–274. doi:10.1126/science.220.4594.268.
Gans, C., & Northcutt, R. G. (1985). Neural crest: The implications for comparative anatomy. Fortschritte der Zoologie, 30, 507–514.
Gass, G., & Hall, B. K. (2007). Collectivity in context: Modularity, cell sociology, and the neural crest. Biological Theory, 2, 1–11. doi:10.1162/biot.2007.2.4.349.
Gavalas, A., Trainor, P., Ariza-McNaughton, L., & Krumlauf, R. (2001). Synergy between Hoxa1 and Hoxb1: The relationship between arch patterning and the generation of cranial neural crest. Development, 128, 3017–3027.
Goodrich, E. S. (1930). Studies on the structure and development of vertebrates. London: Macmillan & Co. Reprinted, 1958, New York: Dover Publications, Inc.; 1986, Chicago: The University of Chicago Press.
Graham, A., & Smith, A. (2001). Patterning the pharyngeal arches. BioEssays, 23, 54–61. doi :10.1002/1521-1878(200101)23:1<54::AID-BIES1007>3.0.CO;2-5.
Grandel, H., Lun, K., Rauch, G.-J., Rhinn, M., et al. (2002). Retinoic acid signalling in the zebrafish embryo is necessary during pre-segmentation stages to pattern the anterior-posterior axis of the CNS and to induce a pectoral fin bud. Development, 129, 2851–2865.
Hall, B. K. (1999a). The neural crest in development and evolution. New York: Springer.
Hall, B. K. (1999b). Evolutionary developmental biology (2nd ed.). Dordrecht, Netherlands: Kluwer Academic Publishers.
Hall, B. K. (2000). A role for epithelial-mesenchymal interactions in tail growth/morphogenesis and chondrogenesis in embryonic mice. Cells, Tissues, Organs, 166, 6–14. doi:10.1159/000016703.
Hall, B. K. (2004). Descent with modification: The unity underlying homology and homoplasy as seen through an analysis of development and evolution. Biological Reviews of the Cambridge Philosophical Society, 78, 409–433. doi:10.1017/S1464793102006097.
Hall, B. K. (2005a). Consideration of the neural crest and its skeletal derivatives in the context of novelty/innovations. Journal of Experimental Zoology. Part B. Molecular and Developmental Evolution, 304B, 548–557.
Hall, B. K. (2005b). Bone and cartilage: Developmental and evolutionary skeletal biology. London: Elsevier Academic Press.
Hall, B. K. (2008a). The neural crest and neural crest cells in vertebrate development and evolution. New York: Springer, in press.
Hall, B. K. (2008b). Vertebrate origins: Riding the crest of a new wave, or the wave of a new crest? Evolution & Development, 10, 261–263.
Hall, B. K. (2008c). The neural crest and neural crest cells: Discovery and significance. Journal of Biosciences, submitted.
Hall, B. K., & Wake, M. H. (Eds.). (1999). The origin and evolution of larval forms. San Diego: Academic Press.
Hammerschmidt, M., Serbedzija, G. N., & McMahon, A. P. (1996). Genetic analysis of dorsoventral pattern formation in the zebrafish: Requirement of a BMP-like ventralizing activity and its dorsal repressor. Genes and Development, 10, 2452–2461. doi:10.1101/gad.10.19.2452.
Hanken, J., & Hall, B. K. (Eds.). (1993). The vertebrate skull (Vol. I–3). Chicago: The University of Chicago Press.
Harada, Y., Okai, N., Taguchi, S., Shoguchi, E., et al. (2001). Embryonic expression of a hemichordate distal-less gene. Zoological Science, 18, 57–61. doi:10.2108/zsj.18.57.
Holland, P. W. H. (1996). Molecular biology of lancelets: Insights into development and evolution. Israel Journal of Zoology, 42, S247–S272.
Holland, P. W. H., & Graham, A. (1995). Evolution of regional identity in the vertebrate nervous system. Perspectives on Developmental Neurobiology, 3, 17–27.
Holland, L. Z., & Holland, N. D. (1996). Expression of AmphiHox-1 and AmphiPax-1 in amphioxus embryos treated with retinoic acid: Insights into evolution and patterning of the chordate nerve cord and pharynx. Development, 122, 1829–1838.
Holland, L. Z., & Holland, N. D. (2001). Evolution of neural crest and placodes: Amphioxus as a model for the ancestral vertebrate? Journal of Anatomy, 199, 85–98.
Holland, N. D., Panganiban, G., Henyey, E. L., & Holland, L. Z. (1996). Sequence and developmental expression of AmphiDll, an amphioxus Distal-less gene transcribed in the ectoderm, epidermis and nervous system: Insights into evolution of craniate forebrain and neural crest. Development, 122, 2911–2920.
Holland, L. Z., Rached, L. A., Tamme, R., Holland, N. D., et al. (2001). Characterization and developmental expression of the amphioxus homolog of Notch (AmphiNotch): Evolutionary conservation of multiple expression domains in amphioxus and vertebrates. Developmental Biology, 232, 493–507. doi:10.1006/dbio.2001.0160.
Holley, S. A., Jackson, P. D., Sasai, Y., Lu, B., et al. (1995). A conserved system for dorsal-ventral patterning in insects and vertebrates involving Sog and Chordin. Nature, 376, 249–253. doi:10.1038/376249a0.
Imai, K., Takada, N., Satoh, N., & Satou, Y. (2000). β-catenin mediates the specification of endoderm cells in ascidian embryos. Development, 127, 3009–3020.
Janvier, P. (2007). Homologies and evolutionary transitions in early vertebrate history. In J. S. Anderson & H.-D. Sues (Eds.), Major transitions in vertebrate evolution (pp. 57–121). Bloomington: Indiana University Press.
Jeffery, W. R. (1997). Evolution of ascidian development. BioScience, 47, 417–425.
Jeffery, W. R. (2007). Chordate ancestry of the neural crest: New insights from ascidians. Seminars in Developmental Biology, 18, 481–491. doi:10.1016/j.semcdb.2007.04.005.
Jeffery, W. R., Strickler, A. G., & Yamamoto, Y. (2004). Migratory neural crest-like cells form body pigmentation in a urochordate embryo. Nature, 431, 696–699. doi:10.1038/nature02975.
Kimura, C., Takeda, N., Suzuki, M., Oshimura, M., et al. (1997). Cis-acting elements conserved between mouse and pufferfish Otx2 genes govern the expression in mesencephalic neural crest cells. Development, 124, 3929–3941.
Kopinke, D., Sasine, J., Swift, J., Stephens, W. Z., & Piotrowski, T. (2006). Retinoic acid is required for endodermal pouch morphogenesis and not for pharyngeal endoderm specification. Developmental Dynamics, 235, 2696–2709.
Kozmik, Z., Holland, N. D., Kalousova, A., Paces, J., et al. (1999). Characterization of an amphioxus paired box gene, AmphiPax2/5/8: Developmental expression patterns in optic support cells, nephridium, thyroid-like structures and pharyngeal gill slits, but not in the midbrain-hindbrain boundary region. Development, 126, 1295–1304.
Kumano, G., & Nishida, H. (2007). Ascidian embryonic development: An emerging model system for the study of cell fate specification in chordates. Developmental Dynamics, 236, 1732–1747. doi:10.1002/dvdy.21108.
Kuratani, S. C. (1997). Spatial distribution of postotic crest cells defines the head/trunk interface of the vertebrate body: Embryological interpretation of peripheral nerve morphology and evolution of the vertebrate head. Anatomy and Embryology, 195, 1–13. doi:10.1007/s004290050020.
Kuratani, S. C. (2004). Evolution of the vertebrate jaw: Comparative embryology and molecular developmental biology reveal the factors behind evolutionary novelty. Journal of Anatomy, 205, 335–347. doi:10.1111/j.0021-8782.2004.00345.x.
Kuratani, S. C. (2005). Cephalic neural crest cells and the evolution of craniofacial structures in vertebrates: Morphological and embryological significance of the premandibular-mandibular boundary. Zoology (Jena, Germany), 108, 13–25. doi:10.1016/j.zool.2004.12.001.
Kuratani, S. C., Kuraku, S., & Murakami, Y. (2002). Lampreys as an evo-devo model: Lessons from comparative embryology and molecular phylogenetics. Genesis (New York, N.Y.), 34, 175–183. doi:10.1002/gene.10142.
Kuratani, S. C., Nobusada, Y., Horigome, N., & Shigetani, Y. (2001). Embryology of the lamprey and evolution of the vertebrate jaw: Insights from molecular and developmental perspectives. Philosophical Transactions of the Royal Society of London. Series B, Biological Sciences, 356, 1615–1632. doi:10.1098/rstb.2001.0976.
Kurokawa, D., Sakurai, Y., Inoue, A., Nakayama, R., et al. (2006). Evolutionary constraint on Otx2 neurectoderm enhancers-deep conservation from skate to mouse and unique divergence in teleost. Proceedings of the National Academy of Sciences of the United States of America, 103, 19350–19355. doi:10.1073/pnas.0604686103.
Lacalli, T. C. (2006). Prospective protochordate homologs of vertebrate midbrain and MHB, with some thoughts on MHB origins. International Journal of Biological Sciences, 2, 104–109.
Lacosta, A. M., Canudas, J., González, C., Muniesa, P., et al. (2007). Pax7 identifies neural crest, chromatophores lineages and pigment stem cells during zebrafish development. The International Journal of Developmental Biology, 51, 327–331. doi:10.1387/ijdb.062217al.
Langeland, J., Tomsa, J. M., Jackman, W. R., Jr, & Kimmel, C. B. (1998). An amphioxus snail gene: Expression in paraxial mesoderm and neural plate suggests a conserved role in patterning the chordate embryo. Development Genes and Evolution, 208, 569–577. doi:10.1007/s004270050216.
Maderson, P. F. A. (Ed.). (1987). Developmental and evolutionary aspects of the neural crest. New York: Wiley.
Maisey, J. G. (1986). Heads and tails: A chordate phylogeny. Cladistics, 2, 201–256.
Mallatt, J. (1984). Early vertebrate evolution: Pharyngeal structure and the origin of gnathostomes. Journal of Zoology, 204, 169–183.
Mallatt, J. (1996). Ventilation and the origin of jawed vertebrates: A new mouth. Zoological Journal of the Linnean Society London, 117, 329–404.
Mallatt, J., & Chen, J.-Y. (2003). Fossil sister group of craniates: Predicted and found. Journal of Morphology, 258, 1–31. doi:10.1002/jmor.10081.
Manni, L., Lane, N. J., Joly, J.-S., Gasparini, F., et al. (2004). Neurogenic and non-neurogenic placodes in ascidians. The Journal of Experimental Zoology, 302B, 483–504. doi:10.1002/jez.b.21013. Mol Dev Evol.
Mansouri, A., Stoykova, A., Torres, M., & Gruss, P. (1996). Dysgenesis of cephalic neural crest derivatives in Pax7 −/− mutant mice. Development, 122, 831–838.
Martinez-Morales, J.-R., Henrich, T., Ramialison, M., & Wittbrodt, J. (2007). New genes in the evolution of the neural crest differentiation program. Genome Biology, 8, R36.1–R36.17.
Matsuo, I., Kuratani, S., Kimura, C., Takeda, N., & Aizawa, S. (1995). Mouse Otx2 functions in the formation and patterning of rostral head. Genes and Development, 9, 2646–2658. doi:10.1101/gad.9.21.2646.
Mazet, F., & Shimeld, S. M. (2005). Molecular evidence from ascidians for the evolutionary origin of vertebrate cranial sensory placodes. The Journal of Experimental Zoology, 304B, 340–346. doi:10.1002/jez.b.21054. Mol Dev Evol.
Meinertzhagen, I. A., & Okamura, Y. (2001). The larval ascidian nervous system: The chordate brain from its small beginnings. Trends in Neurosciences, 24, 401–410. doi:10.1016/S0166-2236(00)01851-8.
Meulemans, D., & Bronner-Fraser, M. (2002). Amphioxus and lamprey Ap-2 genes: Implications for neural crest evolution and migration patterns. Development, 129, 4953–4962.
Meulemans, D., & Bronner-Fraser, M. (2004). Gene-regulatory interactions in neural crest evolution and development. Developmental Cell, 7, 291–299. doi:10.1016/j.devcel.2004.08.007.
Meulemans, D., & Bronner-Fraser, M. (2007). Insights from amphioxus into the evolution of vertebrate cartilage. PLoS ONE, 2(8), e787. doi:10.1371/journal.pone.0000787.
Miya, T., Morita, K., Suzuki, A., Ueno, N., & Satoh, N. (1997). Functional analysis of an ascidian homologue of vertebrate BMP-2/BMP-4 suggests its role in the inhibition of neural fate specification. Development, 124, 5149–5159.
Niederreither, K., Vermot, J., Le Roux, I., Schuhbaur, B., et al. (2003). The regional pattern of retinoic acid synthesis by RALDH2 is essential for the development of posterior pharyngeal arches and the enteric nervous system. Development, 130, 2525–2534. doi:10.1242/dev.00463.
Northcutt, R. G. (1996). The origin of craniates—Neural crest, neurogenic placodes, and homeobox genes. Israel Journal of Zoology, 42, S273–S313.
Northcutt, R. G. (2004). Taste buds: Development and evolution. Brain, Behavior and Evolution, 64, 198–206. doi:10.1159/000079747.
Northcutt, R. G., & Gans, C. (1983). The genesis of neural crest and epidermal placodes: A reinterpretation of vertebrate origins. The Quarterly Review of Biology, 58, 1–28. doi:10.1086/413055.
Nübler-Jung, K., & Arendt, D. (1994). Is ventral in insects dorsal in vertebrates? A history of embryological arguments favouring axis inversion in chordate ancestors. Wilhelm Roux’ Archiv. Developmental Biology, 203, 357–366.
Olsson, L., Ericsson, R., & Cerny, R. (2005). Vertebrate head development: Segmentation, novelties, and homology. Theory in Biosciences, 124, 145–163.
Ota, K. G., Kuraku, S., & Kuratani, S. (2007). Hagfish embryology with reference to the evolution of the neural crest. Nature, 446, 672–675. doi:10.1038/nature05633.
Ota, K. G., & Kuratani, S. (2006). The history of scientific endeavors towards understanding hagfish embryology. Zoological Science, 23, 403–418. doi:10.2108/zsj.23.403.
Ota, K. G., & Kuratani, S. (2007). Cyclostome embryology and early evolutionary history of vertebrates. Integrative and Comparative Biology, 47, 329–337. doi:10.1093/icb/icm022.
Pasini, A., Amiel, A., Rothbacher, U., Roure, A., et al. (2006). Formation of the ascidian epidermal sensory neurons: Insights into the origin of the chordate peripheral nervous system. PLoS Biology, 4, e225. doi:10.1371/journal.pbio.0040225.
Qiu, M., Bulfone, A., Ghattas, I., Meneses, J. J., et al. (1997). Role of the Dlx homeobox genes in proximodistal patterning of the branchial arches: Mutations of Dlx-1, Dlx-2, and Dlx-1 and -2 alter morphogenesis of proximal skeletal and soft tissue structures derived from the first and second arches. Developmental Biology, 185, 165–284. doi:10.1006/dbio.1997.8556.
Raible, D. W., & Ragland, J. W. (2005). Reiterated Wnt and Bmp signals in neural crest development. Seminars in Cell & Developmental Biology, 16, 673–682. doi:10.1016/j.semcdb.2005.06.008.
Rhinn, M., Dierich, A., Shawlot, W., Behringer, R. R., et al. (1998). Sequential roles for Otx2 in visceral endoderm and neurectoderm for forebrain and midbrain induction and specification. Development, 125, 845–856.
Romer, A. S. (1972). The vertebrate as a dual animal-somatic and visceral. Evolutionary Biology, 6, 121–156.
Rychel, A. L., & Swalla, B. J. (2007). Development and evolution of chordate cartilage. The Journal of Experimental Zoology, 308B, 325–335. doi:10.1002/jez.b.21157. Mol Dev Evol.
Sardet, C., Swalla, B. J., Satoh, N., Sasakura, Y., et al. (2008). Euro chordates: Ascidian community swims ahead. The 4th International Tunicate Meeting in Villefranche sur-Mer. Developmental Dynamics, 237, 1207–1213. doi:10.1002/dvdy.21487.
Satoh, N. (1994). Developmental biology of ascidians. Cambridge: Cambridge University Press.
Saudemont, A., Dray, N., Hudry, B., Le Gouar, M., et al. (2008). Complementary striped expression patterns of NK homeobox genes during segment formation in the annelid Platynereis. Developmental Biology, 317, 430–433. doi:10.1016/j.ydbio.2008.02.013.
Sauka-Spengler, T., Meulemans, D., Jones, M., & Bronner-Fraser, M. (2007). Ancient evolutionary origin of the neural crest gene regulatory network. Developmental Cell, 13, 405–420. doi:10.1016/j.devcel.2007.08.005.
Schaeffer, B. (1977). The dermal skeleton in fishes. In S. M. Andrews, R. S. Miles, & A. D. Walker (Eds.), Linnean society symposium # 4 (pp. 25–52). London: Academic Press.
Schlosser, G. (2007). How old genes make a new head: Redeployment of Six and Eya genes during the evolution of vertebrate cranial placodes. Integrative and Comparative Biology, 47, 343–359. doi:10.1093/icb/icm031.
Sharman, A. C., & Holland, P. W. H. (1998). Estimation of Hox gene cluster number in lampreys. The International Journal of Developmental Biology, 42, 617–620.
Shimeld, S. M. (1999). The evolution of dorsoventral pattern formation in the chordate neural tube. American Zoologist, 39, 641–649.
Shu, D.-G., Zhang, X.-L., & Chen, L. (1996). Reinterpretation of Yunnanozoon as the earliest known hemichordate. Nature, 380, 428–430. doi:10.1038/380428a0.
Simeone, A., Acampora, D., Gulisano, M., Stornaiuolo, A., & Boncinelli, E. (1992). Nested expression domains of four homeobox genes in developing rostral brain. Nature, 358, 687–690. doi:10.1038/358687a0.
Smith, M. M., & Hall, B. K. (1990). Developmental and evolutionary origins of vertebrate skeletogenic and odontogenic tissues. Biological Reviews of the Cambridge Philosophical Society, 65, 277–374. doi:10.1111/j.1469-185X.1990.tb01427.x.
Smith, M. M., & Hall, B. K. (1993). A developmental model for evolution of the vertebrate exoskeleton and teeth: The role of cranial and trunk neural crest. Evolutionary Biology, 27, 387–448.
Sperber, S. M., Saxena, V., Hatch, G., & Ekker, M. (2008). Zebrafish dlx2a contributes to hindbrain neural crest survival, is necessary for differentiation of sensory ganglia and functions with dlxa1 in maturation of the arch cartilage elements. Developmental Biology, 314, 59–70. doi:10.1016/j.ydbio.2007.11.005.
Stach, T. (2000). Microscopic anatomy of developmental stages of Branchiostoma lanceolatum (Cephalochordata, Chordata). Bonner zoologische Beiträge, 47, 1–111.
Stone, J. R., & Hall, B. K. (2004). Latent homologues for the neural crest as an evolutionary novelty. Evolution & Development, 6, 123–129. doi:10.1111/j.1525-142X.2004.04014.x.
Suzuki, T., Sakai, D., Osumi, N., Wada, H., & Wakamatsu, Y. (2006). Sox genes regulate type 2 collagen expression in avian neural crest cells. Development, Growth & Differentiation, 48, 477–486. doi:10.1111/j.1440-169X.2006.00886.x.
Wada, H., Saiga, H., Satoh, N., & Holland, P. W. H. (1998). Tripartite organization of the ancestral chordate brain and the antiquity of placodes: Insights from ascidian Pax–2/5/8, Hox and Otx genes. Development, 125, 1113–1122.
Whittaker, J. R. (1987). Cell lineages and determinants of cell fate in development. American Zoologist, 27, 607–622.
Williams, N. A., & Holland, P. W. H. (1998). Gene and domain duplication in the Chordate Otx gene family: Insights from Amphioxus Otx. Molecular Biology and Evolution, 15, 600–607.
Zhang, G., & Cohn, M. J. (2006). Hagfish and lancelet fibrillar collagens reveal that type II collagen-based cartilage evolved in stem vertebrates. Proceedings of the National Academy of Sciences of the United States of America, 103, 16829–16833. doi:10.1073/pnas.0605630103.
Acknowledgments
I thank Ryan Kearney, Daniel Meulemans and the two anonymous reviewers for constructive comments on the paper, an expanded version of which (including additional literature and discussion of the origin of jaws) will appear as Chapter 4 in Hall (2008a). Research support from the Natural Sciences and Engineering Research Council of Canada (grant # A5056) is gratefully acknowledged.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Hall, B.K. Evolutionary Origins of the Neural Crest and Neural Crest Cells. Evol Biol 35, 248–266 (2008). https://doi.org/10.1007/s11692-008-9033-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11692-008-9033-8