Abstract
Aim of the study
The growing resistance of helminth parasites to currently available commercial anthelmintic drugs, combined with apprehensions regarding detrimental chemical residues in livestock products, has sparked an interest in exploring medicinal plants as an alternative strategy for treating helminthiasis. As a result, this study was designed to investigate the anthelmintic activity of crude methanolic extracts (CME) of Saussurea costus root on Ascaridia.galli, a pathogenic nematode of poultry.
Materials and methods
In vitro, the anthelmintic effect of Saussurea costus root was evaluated in comparison to commercial anthelmintic, levamisole on the adult nematode parasites, A.galli using worm motility inhibition (WMI) test. The CME of S.costus was also evaluated for in vivo anthelmintic activity in chickens experimentally infected with Ascaridia galli. For the in vivo study, one hundred-day-old chickens were orally infected with embryonated eggs of A. galli worms. The efficacy of the plant extract as an anthelmintic was assessed through two tests: faecal egg count reduction (FECR) test and worm count reduction (WCR) test. The study investigated three distinct doses of plant extract under in vivo setup: 500 mg kg−1 body weight (bw), 1000 mg kg−1 bw, and 2000 mg kg−1 bw.
Results
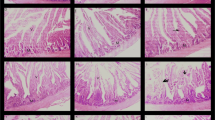
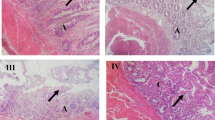
In vitro, all the tested concentrations of S.costus (25 mg/ml, 50 mg/ml, and 100 mg/ml) showed a significant (P < 0.001) anthelmintic effects on live adult A. galli worms in terms of inhibition of worm motility at different hours post-treatment. At the highest concentration of the extract, we observed worm motility inhibition of 100% at 24 h post-exposure. On day 14 post-treatment, all birds were slaughtered, and adult A. galli worms were subsequently retrieved from their small intestines. Birds treated with CME extract of S. costus root exhibited a significant (P < 0.001) reduction in faecal egg count. However, the administration of the extract at the dosage of 500 mg kg−1bw to the birds did not reveal any significant (P > 0.05) differences in the worm count compared to the negative control group. The CME of S. costus at a dose of 2000 mg kg−1bw showed the highest anthelmintic activity by inducing 83.10% FECR and 76.47% WCR.
Conclusion
In conclusion, the root extract of S. costus has a promising anthelmintic activity on A. galli as demonstrated by the results of the present experiment.
Similar content being viewed by others
Data availability
The data supporting this research work can be availed from the corresponding author upon reasonable request.
References
Abdelqader A, Qarallah B, Al-Ramamneh D, Daş G (2012) Anthelmintic effects of citrus peels ethanolic extracts against Ascaridia galli. Vet Parasitol 188:78–84. https://doi.org/10.1016/j.vetpar.2012.03.003
Shifaw A, Feyera T, Walkden-Brown SW, Sharpe B, Elliott T, Ruhnke I (2021) Global and regional prevalence of helminth infection in chickens over time: a systematic review and meta-analysis. Poult Sci 100:101082. https://doi.org/10.1016/j.psj.2021.101082
Permin A, Bisgaard M, Frandsen F, Pearman M, Kold J, Nansen P (1999) Prevalence of gastrointestinal helminths in different poultry production systems. Br Poult Sci 40:439–443. https://doi.org/10.1080/00071669987179
Kaufmann F, Daş G, Sohnrey B, Gauly M (2011) Helminth infections in laying hens kept in organic free range systems in Germany. Livest Sci 141:182–187. https://doi.org/10.1016/j.livsci.2011.05.015
Zhao Y, Lu SF, Li J (2022) Sequence analyses of mitochondrial gene may support the existence of cryptic species within Ascaridia galli. J Helminthol 96:e39. https://doi.org/10.1017/S0022149X2200030X
Poulopoulou I, Horgan MJ, Siewert B, Siller M, Palmieri L, Martinidou E, Gauly M (2022) In vitro evaluation of the effects of methanolic plant extracts on the embryonation rate of Ascaridia galli eggs. Vet Res Commun. https://doi.org/10.1007/s11259-022-09958-9
Daş G, Kaufmann F, Abel H, Gauly M (2010) Effect of extra dietary lysine in Ascaridia galli-infected grower layers. Vet Parasitol 170:238–243. https://doi.org/10.1016/j.vetpar.2010.02.026
Katoch R, Yadav A, Godara R, Khajuria JK, Borkataki S, Sodhi SS (2012) Prevalence and impact of gastrointestinal helminths on body weight gain in backyard chickens in the subtropical and humid zone of Jammu, India. J Parasit Dis 36:49–52. https://doi.org/10.1007/s12639-011-0090-z
Kilpinen O, Roepstorff A, Permin A, Nørgaard-Nielsen G, Lawson LG, Simonsen HB (2005) Influence of Dermanyssus gallinae and Ascaridia galli infections on behaviour and health of laying hens (Gallus gallus domesticus). Br Poult Sci 46:26–34. https://doi.org/10.1080/00071660400023839
Ramadan HH, Znada NYA (1991) Some pathological and biochemical studies on experimental ascaridiasis in chickens. Food Nahrung 35:71–84. https://doi.org/10.1002/food.19910350120
Tarbiat B, Jansson DS, Wall H, Tydén E, Höglund J (2020) Effect of a targeted treatment strategy against Ascaridia galli on egg production, egg quality and bird health in a laying hen farm. Vet Parasitol 286:109238. https://doi.org/10.1016/j.vetpar.2020.109238
Sharma N, Hunt PW, Hine BC, Sharma NK, Swick RA, Ruhnke I (2018) Detection of Ascaridia galli infection in free-range laying hens. Vet Parasitol 256:9–15. https://doi.org/10.1016/j.vetpar.2018.04.009
Hørning G, Rasmussen S, Permin A, Bisgaard M (2003) Investigations on the influence of helminth parasites on vaccination of chickens against Newcastle disease virus under village conditions. Trop Anim Health Prod 35:415–424. https://doi.org/10.1023/A:1025863412078
Pleidrup J, Dalgaard TS, Norup LR, Permin A, Schou TW, Skovgaard K, Juul-Madsen HR (2014) Ascaridia galli infection influences the development of both humoral and cell-mediated immunity after Newcastle Disease vaccination in chickens. Vaccine 32:383–392. https://doi.org/10.1016/j.vaccine.2013.11.034
Collins JB, Jordan B, Baldwin L, Hebron C, Paras K, Vidyashankar AN, Kaplan RM (2019) Resistance to fenbendazole in Ascaridia dissimilis, an important nematode parasite of turkeys. Poult Sci 98:5412–5415. https://doi.org/10.3382/ps/pez379
Ramos F, Portella LP, de Souza RF, Reginato CZ, Pötter L, Cezar AS, Vogel FSF (2016) Anthelmintic resistance in gastrointestinal nematodes of beef cattle in the state of Rio Grande do Sul, Brazil. Int J Parasitol: Drugs and Drug Resist 6:93–101. https://doi.org/10.1016/j.ijpddr.2016.02.002
Patel T, Marmulak T, Gehring R, Pitesky M, Clapham MO, Tell LA (2018) Drug residues in poultry meat: a literature review of commonly used veterinary antibacterials and anthelmintics used in poultry. J Vet Pharmacol Ther 41:761–789. https://doi.org/10.1111/jvp.12700
Cabardo DE Jr, Portugaliza HP (2017) Anthelmintic activity of Moringa oleifera seed aqueous and ethanolic extracts against Haemonchus contortus eggs and third stage larvae. Int J Vet Sci Med. https://doi.org/10.1016/j.ijvsm.2017.02.001
Aziz ARA, AbouLaila MR, Aziz M, Omar MA, Sultan K (2018) In vitro and in vivo anthelmintic activity of pumpkin seeds and pomegranate peels extracts against Ascaridia galli. Beni-Suef Univ J of Basic Appl Sci 7:231–234. https://doi.org/10.1016/j.bjbas.2018.02.003
Zaman MA, Abbas RZ, Qamar W, Qamar MF, Mehreen U, Shahid Z, Kamran M (2020) Role of secondary metabolites of medicinal plants against Ascaridia galli. World’s Poult Sci J 76:639–655. https://doi.org/10.1080/00439339.2020.1782801
Pandey MM, Rastogi S, Rawat AKS (2007) Saussurea costus: Botanical, chemical and pharmacological review of an ayurvedic medicinal plant. J Ethnopharmacol 110:379–390. https://doi.org/10.1016/j.jep.2006.12.033
Madhuri K, Elango K, Ponnusankar S (2012) Saussurea lappa (Kuth root): review of its traditional uses, phytochemistry and pharmacology. Orient Pharm Exp Med 12:1–9. https://doi.org/10.1016/j.jep.2006.12.033
Gairola S, Sharma J, Bedi YS (2014) A cross-cultural analysis of Jammu, Kashmir and Ladakh (India) medicinal plant use. J Ethnopharmacol 155:925–986. https://doi.org/10.1016/j.jep.2014.06.029
Yang HJ, Kim MJ, Kang S, Moon NR, Kim DS, Lee NR, Park S (2017) Topical treatments of Saussurea costus root and Thuja orientalis L. synergistically alleviate atopic dermatitis-like skin lesions by inhibiting protease-activated receptor-2 and NF-κB signaling in HaCaT cells and Nc/Nga mice. J Ethnopharmacol 199:97–105. https://doi.org/10.1016/j.jep.2017.01.055
Mathew M, Subramanian S (2014) In vitro screening for anti-cholinesterase and antioxidant activity of methanolic extracts of ayurvedic medicinal plants used for cognitive disorders. PLoS ONE 9:e86804. https://doi.org/10.1371/journal.pone.0086804
Yaeesh S, Jamal Q, Shah AJ, Gilani AH (2010) Antihepatotoxic activity of Saussurea lappa extract on D-galactosamine and lipopolysaccharide-induced hepatitis in mice. Phytother Res 24:S229–S232. https://doi.org/10.1002/ptr.3089
Moon SM, Yun SJ, Kook JK, Kim HJ, Choi MS, Park BR, Kim CS (2013) Anticancer activity of Saussurea lappa extract by apoptotic pathway in KB human oral cancer cells. Pharm Biol 51:1372–1377. https://doi.org/10.3109/13880209.2013.792847
Liu ZL, He Q, Chu SS, Wang CF, Du SS, Deng ZW (2012) Essential oil composition and larvicidal activity of Saussurea lappa roots against the mosquito Aedes albopictus (Diptera: Culicidae). Parasitol Res 110:2125–2130. https://doi.org/10.1007/s00436-011-2738-0
Rao KS, Babu GV, Ramnareddy YV (2007) Acylated flavone glycosides from the roots of Saussurea lappa and their antifungal activity. Molecules 12:328–344. https://doi.org/10.3390/12030328
Sagar A, Chauhan V, Prakash V (2017) Studies on endophytes and antibacterial activity of Saussurea costus (falc.). J Drug Deliver Thera 7:5–10. https://doi.org/10.22270/jddt.v7i2.1374
Shaikh JR, Patil M (2020) Qualitative tests for preliminary phytochemical screening: an overview. Int J Chem Stud 8(2):603–608. https://doi.org/10.22271/chemi.2020.v8.i2i.8834
Tariq KA, Chishti MZ, Ahmad F, Shawl AS (2009) Anthelmintic activity of extracts of Artemisia absinthium against ovine nematodes. Vet Parasitol 160:83–88. https://doi.org/10.1016/j.vetpar.2008.10.084
Feyera T, Ruhnke I, Sharpe B, Elliott T, Campbell DLM, Walkden-Brown SW (2020) Viability and development of Ascaridia galli eggs recovered in artificial media followed by storage under different conditions. J Helminthol 94:e199. https://doi.org/10.1017/S0022149X2000084X
Yazwinski TA, Chapman HD, Davis RB, Letonja T, Pote L, Maes L, Jacobs DE (2003) World association for the advancement of veterinary parasitology (WAAVP) guidelines for evaluating the effectiveness of anthelmintics in chickens and turkeys. Vet Parasitol 116:159–173. https://doi.org/10.1016/S0304-4017(03)00264-4
Permin A, Hansen JW (1998) Epidemiology, diagnosis and control of poultry parasites. FAO animal health manuals 4. Food and Agriculture Organization of the United Nations (FAO), Rome, p 160
Oju JPE, Mpoame M (2006) Periodic release of gastrointestinal helminth eggs in native chickens from Dschang in the western highlands of Cameroon. Vet Res commun 30:39–43. https://doi.org/10.1007/s11259-005-3159-2
Coles GC, Bauer C, Borgsteede FHM, Geerts S, Klei TR, Taylor MA, Waller PJ (1992) World association for the advancement of veterinary parasitology (WAAVP) methods for the detection of anthelmintic resistance in nematodes of veterinary importance. Vet Parasitol 44:35–44. https://doi.org/10.1016/0304-4017(92)90141-U
Grzybek M, Kukula-Koch W, Strachecka A, Jaworska A, Phiri AM, Paleolog J, Tomczuk K (2016) Evaluation of anthelmintic activity and composition of pumpkin (Cucurbita pepo L.) seed extracts—in vitro and in vivo studies. Int J Mol Sci 17:1456. https://doi.org/10.3390/ijms17091456
Imani-Baran A, Abdollahi J, Akbari H, Jafarirad S, Moharramnejad S (2020) Anthelmintic activity of crude powder and crude aqueous extract of Trachyspermum ammi on gastrointestinal nematodes in donkey (Equus asinus): An in vivo study. J ethnopharmacol 248:112249. https://doi.org/10.1016/j.jep.2019.112249
Bazh EK, El-Bahy NM (2013) In vitro and in vivo screening of anthelmintic activity of ginger and curcumin on Ascaridia galli. Parasitol Res 112:3679–3686. https://doi.org/10.1007/s00436-013-3541-x
Lalchhandama K, Roy B, Dutta BK (2009) Anthelmintic activity of Acacia oxyphylla stem bark against Ascaridia galli. Pharma Biol 47:578–583. https://doi.org/10.1080/13880200902902463
Mazhangara IR, Masika PJ, Mupangwa JF, Chivandi E, Jaja IF, Muchenje V (2020) In vitro efficacy of Elephantorrhiza elephantina root extracts against adult Paramphistomum cervi in goats. Parasite Epidemiol Control 10:e00157. https://doi.org/10.1016/j.parepi.2020.e00157
Gasaliyu KA, Ajanusi OJ, Suleiman MM, Dahiru S, Yusuf KH, Kyari S, Orakpoghenor O (2022) Effects of Vernonia amygdalina methanol leaf extract and fractions on Ascaridia galli in experimentally infected birds with regard to its pathological effect. Bull Natl Res Cent 46:131. https://doi.org/10.1186/s42269-022-00819-8
Nghonjuyi NW, Keambou CT, Sofeu-Feugaing DD, Taiwe GS, Aziz ARA, Lisita F, Kimbi HK (2020) Mimosa pudica and Carica papaya extracts on Ascaridia galli-experimentally infected kabir chicks in cameroon: efficacy, lipid and hematological profile. Vet Parasitol: Reg Studi Report 19:100354. https://doi.org/10.1016/j.vprsr.2019.100354
Davuluri T, Chennuru S, Pathipati M, Krovvidi S, Rao GS (2020) In vitro anthelmintic activity of three tropical plant extracts on Haemonchus contortus. Acta Parasitol 65:11–18. https://doi.org/10.2478/s11686-019-00116-x
Hossain E, Chandra G, Nandy AP, Mandal SC, Gupta JK (2012) Anthelmintic effect of a methanol extract of leaves of Dregea volubilis on Paramphistomum explanatum. Parasitol Res 110:809–814. https://doi.org/10.1007/s00436-011-2558-2
Stephen K, Ajanusi OJ, Suleiman MM, Orakpoghenor O, Ogwiji M (2022) In vitro and in vivo anthelmintic effects of Sterospermum kunthianum (Cham-Holl) leaf extract against Ascaridia galli in experimentally infected broiler chickens. J Parasit Dis 46:152–158. https://doi.org/10.1007/s12639-021-01426-6
Williams AR, Fryganas C, Ramsay A, Mueller-Harvey I, Thamsborg SM (2014) Direct anthelmintic effects of condensed tannins from diverse plant sources against Ascaris suum. PLoS ONE 9:e97053. https://doi.org/10.1371/journal.pone.0097053
Hernandez PM, Salem AZ, Elghandour MM, Cipriano-Salazar M, Cruz-Lagunas B, Camacho LM (2014) Anthelmintic effects of Salix babylonica L. and Leucaena leucocephala Lam. extracts in growing lambs. Trop Anim Health Prod 46:173–178. https://doi.org/10.1007/s11250-013-0471-7
Hussein D, El-Shiekh RA, Saber FR, Attia MM, Mousa MR, Atta AH, Mouneir SM (2021) Unravelling the anthelmintic bioactives from Jasminum grandiflorum L. subsp. Floribundum adopting in vitro biological assessment. J Ethnopharmacol 275:114083. https://doi.org/10.1016/j.jep.2021.114083
Acknowledgements
We are grateful to the Department of Zoology (University of Kashmir, India) for providing infrastructural support.
Funding
No funding was received for conducting this research work.
Author information
Authors and Affiliations
Contributions
Fayaz Hussain Mir: Conceptualization, Methodology, Experimental work, Writing. Syed Tanveer: Conceptualization, Methodology, Supervision, Reviewing. Pooja Bharti: Experimental work, Writing. Bilal Ahmad Para: Statistical analysis, Reviewing. Corresponding author. Correspondence to Fayaz Hussain Mir.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflicts of interest in relation to this work.
Ethical approval
This experimental study was authorized by the Institutional Ethical Committee (IAEC), Department of Pharmaceutical Sciences (University of Kashmir) under approval number: F(IAEC-permission) KU/2021/03.
Consent to participate
Not applicable.
Consent to publish
Not applicable.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Mir, F.H., Tanveer, S., Bharti, P. et al. Anthelmintic Activity of Saussurea costus (Falc.) Lipsch. Against Ascaridia galli, a Pathogenic Nematode in Poultry: In Vitro and In Vivo Studies. Acta Parasit. (2024). https://doi.org/10.1007/s11686-024-00837-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11686-024-00837-8