Abstract
Background
Piroplasmosis and anaplasmosis stand out as the primary diseases affecting livestock during periods of tick activity. These vector-borne diseases continue to emerge worldwide, exerting a detrimental impact on both animal health and national economies. The purpose of this study is to assess the prevalence of Piroplasma spp. and its co-occurrence with Anaplasma marginale in domestic ruminants in Algeria.
Methods
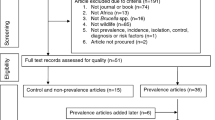
Three databases were systematically reviewed to identify eligible studies for the final meta-analysis, following the PRISMA statement. The 'meta' package in the R software was employed for the meta-analysis with the random effects model chosen for data pooling.
Results
The meta-analysis encompasses 14 research papers spanning a 19-year period (2004–2023). Theileria spp. was identified in all studies, covering 1675 cattle, 190 sheep, and 128 goats, yielding an overall Theileria infection rate of 45% (95% CI 26–65%). Specifically, cattle had a 59% infection rate, while sheep and goats had rates of 18% and 20%, respectively. Babesia spp. was found in nine studies, involving 1183 cattle and 190 sheep, resulting in an overall Babesia infection rate of 7% (95% CI 4–15%), with cattle and sheep having rates of 10% and 3%, respectively. Notably, eight Piroplasma species T. annulata, T. orientalis, T. buffeli, T. equi, Theileria sp., B. bovis, B. bigemina, and B. occultans were detected in cattle, with T. annulata being the most prevalent at 54%. Regional disparities and host factors also impacted infection rates, with higher rates in Northeastern Algeria and among suspected disease cattle. Additionally, gender, age, and breed influenced cattle susceptibility to Theileria infection. Furthermore, six distinct co-infections between Piroplasma spp. and A. marginale were observed, with T. annulata/A. marginale identified in six studies, demonstrating an 8.3% co-infection rate.
Conclusion
This analysis offers crucial insights into the current status of Piroplasmosis and its co-infection with A. marginale in Algerian domestic ruminants, providing valuable data for surveillance and prevention strategies.





Similar content being viewed by others
Data availability
All the relevant data are available in the manuscript.
References
Mehlhorn H, (2016) Protozoan Parasites. In: Anim. Parasites. Springer International Publishing, Cham, pp 33–249. https://doi.org/10.1007/978-3-319-46403-9_4
Chauvin A, Moreau E, Bonnet S, Plantard O, Malandrin L (2009) Babesia and its hosts: adaptation to long-lasting interactions as a way to achieve efficient transmission. Vet Res 40:37. https://doi.org/10.1051/vetres/2009020
Semara L, Mouffok C, Madani T (2013) Livestock farming systems and cattle production orientation in eastern high plains of algeria, cattle farming system in Algerian semi arid Region. Int J Agric Manag Dev 3:237–244
Mouffok C, Allouni A, Semara L, Belkasmi F (2019) Factors affecting the conception rate of artificial insemination in small cattle dairy farms in an Algerian semi-arid area. Livest Res Rural Dev 31:1–9
Kardjadj M, Kouidri B, Metref D, Luka PD, Ben-Mahdi MH (2016) Abortion and various associated risk factors in small ruminants in Algeria. Prev Vet Med 123:97–101. https://doi.org/10.1016/j.prevetmed.2015.11
MADR, (2014) Ministère de l’Agriculture et de Développement Rural, Algérie. Annuel rapport. madr.gov.dz
MADR, (2021) statistiques agricoles,Ministère de l’Agriculture et du Développement Rural, statistiques agricoles, madr.gov.dz.
Ayadi O, Gharbi M, Benchikh Elfegoun MC (2016) Milk losses due to bovine tropical theileriosis (Theileria annulata infection) in Algeria. Asian Pac J Trop Biomed 6:801–802. https://doi.org/10.1016/j.apjtb.2016.06.014
Elfegoun MCB, Gharbi M, Merzekani Z, Kohil K (2017) Piroplasmoses bovines dans les provinces de Skikda et d’Oum El Bouaghi (nord-est de l’Algérie): étude épidémiologique et estimation des pertes de production laitière. Rev d’élevage médecine vétérinaire des pays Trop 70:105–110
Gebrekidan H, Perera PK, Ghafar A, Abbas T, Gasser RB, Jabbar A (2020) An appraisal of oriental theileriosis and the Theileria orientalis complex, with an emphasis on diagnosis and genetic characterisation. Parasitol Res 119:11–22. https://doi.org/10.1007/s00436-019-06557-7
Abdelbaset AE, Nonaka N, Nakao R, (2022) Tick-borne diseases in Egypt: A one health perspective. One Heal 100443. https://doi.org/10.1016/j.onehlt.2022.100443
Chen Y, Chen Y-Y, Liu G, Lyu C, Hu Y, An Q, Qiu H-Y, Zhao Q, Wang C-R (2022) Prevalence of Theileria in cattle in China: a systematic review and meta-analysis. Microb Pathog 162:105369. https://doi.org/10.1016/j.micpath.2021.105369
Mans BJ, Pienaar R, Latif AA (2015) A review of Theileria diagnostics and epidemiology. Int J Parasitol Parasites Wildl 4:104–118. https://doi.org/10.1016/j.ijppaw.2014.12.006
Morrison WI (2015) The aetiology, pathogenesis and control of theileriosis in domestic animals. Rev Sci Tech 34:599–611. https://doi.org/10.20506/rst.34.2.2383
Amira A-H, Ahmed L, Ahmed J, Nijhof A, Clausen P-H (2018) Epidemiological study on tropical theileriosis (Theileria annulata infection) in the Egyptian Oases with special reference to the molecular characterization of Theileria spp. Ticks Tick Borne Dis 9:1489–1493. https://doi.org/10.1016/j.ttbdis.2018.07.008
Kiara H, Steinaa L, Nene V, Svitek N (2018) Theileria in ruminants. Parasit protozoa farm Anim pets 187–213. https://doi.org/10.1007/978-3-319-70132-5_8
Ziam H, Kelanamer R, Aissi M, Ababou A, Berkvens D, Geysen D (2015) Prevalence of bovine theileriosis in North Central region of Algeria by real-time polymerase chain reaction with a note on its distribution. Trop Anim Health Prod 47:787–796. https://doi.org/10.1007/s11250-015-0772-0
Ziam H, Saidani K, Aissi M (2017) Prevalence of bovine piroplasmosis and anaplasmosis in north-central Algeria. Sci Parasitol 18:7–15
Ziam H, Benaouf H (2004) Prevalence of blood parasites in cattle from wilayates of Annaba and El Tarf east Algeria. Arch Inst Pasteur Tunis 81:27–30
Dib L, Bitam I, Tahri M, Bensouilah M, De Meeûs T (2008) Competitive exclusion between piroplasmosis and anaplasmosis agents within cattle. PLoS Pathog 4:2–5. https://doi.org/10.1371/journal.ppat.0040007
Boularias G, Azzag N, Gandoin C, Bouillin C, Chomel B, Haddad N, Boulouis H-J (2020) Bovines harbor a diverse array of vector-borne pathogens in Northeast Algeria. Pathogens 9:1–13. https://doi.org/10.3390/pathogens9110883
Aparna M, Ravindran R, Vimalkumar MB, Lakshmanan B, Rameshkumar P, Kumar KGA, Promod K, Ajithkumar S, Ravishankar C, Devada K (2011) Molecular characterization of Theileria orientalis causing fatal infection in crossbred adult bovines of South India. Parasitol Int 60:524–529. https://doi.org/10.1016/j.parint.2011.08.002
Perera PK, Gasser RB, Firestone SM, Anderson GA, Malmo J, Davis G, Beggs DS, Jabbar A (2014) Oriental theileriosis in dairy cows causes a significant milk production loss. Parasit Vectors 7:1–8. https://doi.org/10.1186/1756-3305-7-73
Aouadi A, Leulmi H, Boucheikhchoukh M, Benakhla A, Raoult D, Parola P (2017) Molecular evidence of tick-borne hemoprotozoan-parasites (Theileria ovis and Babesia ovis) and bacteria in ticks and blood from small ruminants in Northern Algeria. Comp Immunol Microbiol Infect Dis 50:34–39. https://doi.org/10.1016/j.cimid.2016.11.008
Sadeddine R, Diarra AZ, Laroche M, Mediannikov O, Righi S, Benakhla A, Dahmana H, Raoult D, Parola P, (2020) Molecular identification of protozoal and bacterial organisms in domestic animals and their infesting ticks from north-eastern Algeria. Ticks Tick Borne Dis. https://doi.org/10.1016/j.ttbdis.2019.101330
Naz S, Maqbool A, Ahmed S, Ashraf K, Ahmed N, Saeed K, Latif M, Iqbal J, Ali Z, Shafi K (2012) Prevalence of theileriosis in small ruminants in Lahore-Pakistan. J Vet Anim Sci 2:16–20
Khan A, Muhammed AA, Nasreen N, Iqbal F, Cossio-Bayugar R, ali Sha SS, Alanazi AD, Zajac Z, (2022) Tick-borne haemoparasitic diseases in small ruminants in Pakistan: Current knowledge and future perspectives. Saudi J Biol Sci 29:2014–2025. https://doi.org/10.1016/j.sjbs.2021.12.046
Bock R, Jackson L, De Vos A, Jorgensen W (2004) Babesiosis of cattle. Parasitology 129:S247–S269
Mehlhorn H, (2016) Encyclopedia of Parasitology. Encycl Parasitol Springer, Berlin, Heidelb. https://doi.org/10.1007/978-3-662-43978-4
Ziam H, Kernif T, Saidani K, Kelanemer R, Hammaz Z, Geysen D (2020) Bovine piroplasmosis-anaplasmosis and clinical signs of tropical theileriosis in the plains of Djurdjura (north Algeria). Vet Med Sci 6:720–729. https://doi.org/10.1002/vms3.305
Foughali AA, Mhadhbi M, Amairia S, Dhibi M, Bitam I, Boukabache H, Berbar A, Rjeibi MR, Gharbi M (2023) Cattle co-infection patterns by hemopathogens and their phylogenetic analysis during the tick season in Constantine and Mila. Parasitol Res, Northeast Algeria. https://doi.org/10.1007/s00436-023-07916-1
Yeruham I, Hadani A, Galker F (1998) Some epizootiological and clinical aspects of ovine babesiosis caused by Babesia ovis—a review. Vet Parasitol 74:153–163. https://doi.org/10.1016/S0304-4017(97)00143-X
Stuen S (2016) Haemoparasites in small ruminants in European countries: Challenges and clinical relevance. Small Rumin Res 142:22–27. https://doi.org/10.1016/j.smallrumres.2016.03.005
Stuen S (2020) Haemoparasites—Challenging and wasting infections in small ruminants: A review. Animals 10:2179. https://doi.org/10.3390/ani10112179
Hashemi-Fesharki R (1997) Tick-borne diseases of sheep and goats and their related vectors in Iran. Parassitologia 39:115–117
Meliani P, Khatibi S, Randazzo S, Gorenflot A, Marchou B (2006) Human babesiosis. Med Mal Infect 36:499–504. https://doi.org/10.1016/j.medmal.2006.07.002
Foughali AA, Jedidi M, Dhibi M, Mhadhbi M, Sassi L, Berber A, Bitam I, Gharbi M (2021) Infection by haemopathogens and tick infestation of sheep during summer season in Constantine region, Northeast Algeria. Vet Med Sci 7:1769–1777. https://doi.org/10.1002/vms3.551
Kocan KM, De la Fuente J, Guglielmone AA, Meléndez RD (2003) Antigens and alternatives for control of Anaplasma marginale infection in cattle. Clin Microbiol Rev 16:698–712. https://doi.org/10.1128/cmr.16.4.698-712.2003
Sonenshine DE (2018) Range expansion of tick disease vectors in North America: implications for spread of tick-borne disease. Int J Environ Res Publ Health 15:478. https://doi.org/10.3390/ijerph15030478
Boucheikhchoukh M, Laroche M, Aouadi A, Dib L, Benakhla A, Raoult D, Parola P (2018) MALDI-TOF MS identification of ticks of domestic and wild animals in Algeria and molecular detection of associated microorganisms. Comp Immunol Microbiol Infect Dis 57:39–49. https://doi.org/10.1016/j.cimid.2018.05.002
Boularias G, Azzag N, Galon C, Šimo L, Boulouis H-J, Moutailler S (2021) High-throughput microfluidic real-time PCR for the detection of multiple microorganisms in Ixodid cattle ticks in Northeast Algeria. Pathogens 10:362. https://doi.org/10.3390/pathogens10030362
Mechouk N, Mihalca AD, Deak G, Bouslama Z (2022) Synopsis of the ticks of Algeria with new hosts and localities records. Parasit Vectors 15:302. https://doi.org/10.1186/s13071-022-05424-2
Lean IJ, Rabiee AR, Duffield TF, Dohoo IR (2009) Invited review: Use of meta-analysis in animal health and reproduction: Methods and applications. J Dairy Sci 92:3545–3565. https://doi.org/10.3168/jds.2009-2140
Moola S, Munn Z, Sears K, Sfetcu R, Currie M, Lisy K, Tufanaru C, Qureshi R, Mattis P, Mu P (2015) Conducting systematic reviews of association (etiology): the Joanna Briggs Institute’s approach. JBI Evid Implement 13:163–169. https://doi.org/10.1097/xeb.0000000000000064
Dekkers OM, Vandenbroucke JP, Cevallos M, Renehan AG, Altman DG, Egger M (2019) COSMOS-E: guidance on conducting systematic reviews and meta-analyses of observational studies of etiology. PLoS Med 16:e1002742. https://doi.org/10.1371/journal.pmed.1002742
Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, Shamseer L, Tetzlaff JM, Akl EA, Brennan SE (2021) The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. Int J Surg 88:105906. https://doi.org/10.1136/bmj.n71
Ferreira GCM, Canozzi MEA, Peripolli V, de Paula Moura G, Sánchez J, Martins CEN (2022) Prevalence of bovine Babesia spp., Anaplasma marginale, and their co-infections in Latin America: systematic review-meta-analysis. Ticks Tick Borne Dis 13:101967. https://doi.org/10.1016/j.ttbdis.2022.101967
Paramanandham K, Mohankumar A, Suresh KP, Jacob SS, Roy P (2019) Prevalence of Anaplasma species in India and the World in dairy animals: a systematic review and meta-analysis. Res Vet Sci 123:159–170. https://doi.org/10.1016/j.rvsc.2019.01.013
Krishnamoorthy P, Akshata LG, Jacob SS, Suresh KP, Roy P (2021) Theileriosis prevalence status in cattle and buffaloes in India established by systematic review and meta-analysis. Indian J Anim Sci. https://doi.org/10.56093/ijans.v91i4.114331
Borenstein M, Hedges LV, Higgins JPT, Rothstein HR (2010) A basic introduction to fixed-effect and random-effects models for meta-analysis. Res Synth Methods 1:97–111. https://doi.org/10.1002/jrsm.12
Haghi MM, Etemadifar F, Fakhar M, Teshnizi SH, Soosaraei M, Shokri A, Hajihasani A, Mashhadi H (2017) Status of babesiosis among domestic herbivores in Iran: a systematic review and meta-analysis. Parasitol Res 116:1101–1109. https://doi.org/10.1007/s00436-016-5368-8
Higgins J, Thompson S, Deeks J, Altman D (2002) Statistical heterogeneity in systematic reviews of clinical trials: a critical appraisal of guidelines and practice. J Health Serv Res Policy 7:51–61. https://doi.org/10.1258/1355819021927674
Higgins JPT, Thompson SG, Deeks JJ, Altman DG (2003) Measuring inconsistency in meta-analyses. BMJ 327:557–560. https://doi.org/10.1136/bmj.327.7414.557
Egger M, Smith GD, Schneider M, Minder C (1997) Bias in meta-analysis detected by a simple, graphical test. BMJ 315:629–634. https://doi.org/10.1136/bmj.315.7109.629
Duval S, Tweedie R (2000) Trim and fill: a simple funnel-plot–based method of testing and adjusting for publication bias in meta-analysis. Biometrics 56:455–463. https://doi.org/10.1111/j.0006-341x.2000.00455.x
Soosaraei M, Haghi MM, Etemadifar F, Fakhar M, Teshnizi SH, Hezarjaribi HZ, Asfaram S (2018) Status of theileriosis among herbivores in Iran: a systematic review and meta-analysis. Vet World 11:332. https://doi.org/10.14202/vetworld.2018.332-341
Tawana M, Onyiche TE, Ramatla T, Mtshali S, Thekisoe O (2022) Epidemiology of ticks and tick-borne pathogens in domestic ruminants across Southern African development community (SADC) region from 1980 until 2021: systematic review and meta-analysis. Pathogens 11:929. https://doi.org/10.3390/pathogens11080929
Jia L, Zhao S, Xie S, Li H, Wang H, Zhang S (2020) Molecular prevalence of Theileria infections in cattle in Yanbian, north-eastern China. Parasite 27:19. https://doi.org/10.1051/parasite/2020017
Estrada-Peña A, Santos-Silva MM (2005) The distribution of ticks (Acari: Ixodidae) of domestic livestock in Portugal. Exp Appl Acarol 36:233–246. https://doi.org/10.1007/s10493-005-5107-9
Asmare K, Abayneh T, Sibhat B, Shiferaw D, Szonyi B, Krontveit RI, Skjerve E, Wieland B (2017) Major vectors and vector-borne diseases in small ruminants in Ethiopia: a systematic review. Acta Trop 170:95–104. https://doi.org/10.1016/j.actatropica.2017.02.015
Mushtaq A, Shoukat T, Mumtaz T, Qasim M, Ajmal K, Fatima N, Khan A, Kouser M, Hussain N, Khan SS (2021) Tick-borne diseases in sheep and goats in Pakistan: a systematic review and meta-analysis. Acta Parasitol 66:1316–1325. https://doi.org/10.1007/s11686-021-00396-2
Weny G, Okwee-Acai J, Okech SG, Tumwine G, Ndyanabo S, Abigaba S, Goldberg TL (2017) Prevalence and risk factors associated with hemoparasites in cattle and goats at the edge of Kibale National Park, Western Uganda. J Parasitol 103:69–74. https://doi.org/10.1645/16-33
Jacob SS, Sengupta PP, Krishnamoorthy P, Suresh KP, Patil SS, Chandu AGS, Chamuah JK, Lalrinkima H, Shome BR (2022) Bovine babesiosis in India: Estimation of prevalence by systematic review and meta analysis. Exp Parasitol 239:108318. https://doi.org/10.1016/j.exppara.2022.108318
Jacob SS, Sengupta PP, Paramanandham K, Suresh KP, Chamuah JK, Rudramurthy GR, Roy P (2020) Bovine babesiosis: an insight into the global perspective on the disease distribution by systematic review and meta-analysis. Vet Parasitol 283:109136. https://doi.org/10.1016/j.vetpar.2020.109136
Mohammad Al-Saeed AT, Omer LT, Abdo J, Habibi G, Salih DA, Seitzer U, Ahmed J (2010) Epidemiological studies on tropical theileriosis (Theileria annulata infection of cattle) in Kurdistan Region, Iraq. Parasitol Res 106:403–407. https://doi.org/10.1007/s00436-009-1675-7
El Damaty HM, Yousef SG, El-Balkemy FA, Nekouei O, Mahmmod YS, Elsohaby I (2022) Seroprevalence and risk factors of tropical theileriosis in smallholder asymptomatic large ruminants in Egypt. Front Vet Sci 9:1004378. https://doi.org/10.3389/fvets.2022.1004378
McFadzean H, Johnson N, Phipps LP, Swinson V, Boden LA (2023) Surveillance and risk analysis for bovine babesiosis in england and wales to inform disease distribution. Animals 13:2118. https://doi.org/10.3390/ani13132118
Almería S, Castella J, Ferrer D, Ortuno A, Estrada-Peña A, Gutierrez JF (2001) Bovine piroplasms in Minorca (Balearic Islands, Spain): a comparison of PCR-based and light microscopy detection. Vet Parasitol 99:249–259. https://doi.org/10.1016/s0304-4017(01)00464-2
Kirvar E, Ilhan T, Katzer F, Wilkie G, Hooshmand-Rad P, Brown D (1998) Detection of Theileria lestoquardi (hirci) in Ticks, Sheep, and Goats Using the Polymerase Chain Reaction a. Ann N Y Acad Sci 849:52–62. https://doi.org/10.1111/j.1749-6632.1998.tb11033.x
Narimani B, Hoghooghi-Rad N, Shayan P, Rahbari S (2017) Molecular and microscopic detection of Theileria spp. among cattle and buffaloes in West Azarbaijan, Iran. Arch Razi Inst 72:189–195. https://doi.org/10.22092/ari.2017.111605
García-Bocanegra I, Arenas-Montes A, Hernández E, Adaszek Ł, Carbonero A, Almería S, Jaén-Téllez JA, Gutiérrez-Palomino P, Arenas A (2013) Seroprevalence and risk factors associated with Babesia caballi and Theileria equi infection in equids. Vet J 195:172–178. https://doi.org/10.1016/j.tvjl.2012.06.012
Mohammed-Ahmed GM, Hassan SM, El Hussein AM, Salih DA (2018) Molecular, serological and parasitological survey of Theileria annulata in North Kordofan State, Sudan. Vet Parasitol Reg Stud Reports 13:24–29. https://doi.org/10.1016/j.vprsr.2018.03.006
Mohmad A, Chandra D, Saravanan BC, Manjunathchar HV, OR VK, Fular A, Chigure G, Kaur N, Ghosh S (2018) Development of a recombinant TaSP-based Dot-ELISA for detection of Theileria annulata infection in cattle. Ticks Tick Borne Dis 9:1416–1420. https://doi.org/10.1016/j.ttbdis.2018.06.016
Elhachimi L, Rogiers C, Casaert S, Fellahi S, Van Leeuwen T, Dermauw W, Valcárcel F, Olmeda ÁS, Daminet S, Khatat SEH, Sahibi H, Duchateau L (2021) Ticks and tick-borne pathogens abound in the cattle population of the rabat-sale Kenitra Region. Morocco Pathogens 10:1594. https://doi.org/10.3390/pathogens10121594
M’ghirbi Y, Hurtado A, Barandika JF, Khlif K, Ketata Z, Bouattour A (2008) A molecular survey of Theileria and Babesia parasites in cattle, with a note on the distribution of ticks in Tunisia. Parasitol Res 103:1003–1003. https://doi.org/10.1007/s00436-008-0995-3
AL-Hosary A, Răileanu C, Tauchmann O, Fischer S, Nijhof AM, Silaghi C (2020) Epidemiology and genotyping of Anaplasma marginale and co-infection with piroplasms and other Anaplasmataceae in cattle and buffaloes from Egypt. Parasit Vectors 13:495. https://doi.org/10.1186/s13071-020-04372-z
Abdel-Shafy S, Abdullah HHAM, Elbayoumy MK, Elsawy BSM, Hassan MR, Mahmoud MS, Hegazi AG, Abdel-Rahman EH (2022) Molecular epidemiological investigation of piroplasms and anaplasmataceae bacteria in Egyptian domestic animals and associated ticks. Pathogens 11:1194. https://doi.org/10.3390/pathogens11101194
Alanazi AD, Alouffi AS, Alshahrani MY, Alyousif MS, Abdullah HHAM, Allam AM, Elsawy BSM, Abdel-Shafy S, Alsulami MN, Khan A (2021) A report on tick burden and molecular detection of tick-borne pathogens in cattle blood samples collected from four regions in Saudi Arabia. Ticks Tick Borne Dis 12:101652. https://doi.org/10.1016/j.ttbdis.2021.101652
Asif M, Ben SM, Parveen A, Ejaz A, Ikram M, Awais MM, Ozubek S, Aktas M, Baber M, Iqbal F (2022) Seasonal survey, risk factor’s analysis and genotyping of Theileria annulata infecting cattle in Punjab province. Pakistan Acta Trop 234:106587. https://doi.org/10.1016/j.actatropica.2022.106587
Naik BS, Maiti SK, Raghuvanshi PDS (2016) Prevalence of tropical theileriosis in cattle in Chhattisgarh state. J Anim Res 6:1043. https://doi.org/10.5958/2277-940X.2016.00151.0
Dumanli N, Aktas M, Cetinkaya B, Cakmak A, Koroglu E, Saki CE, Erdogmus Z, Nalbantoglu S, Ongor H, Simşek S, Karahan M, Altay K (2005) Prevalence and distribution of tropical theileriosis in Eastern Turkey. Vet Parasitol 127:9–15. https://doi.org/10.1016/j.vetpar.2004.08.006
Almería S, Delgado-Neira Y, Adelantado C, Huguet M, Vinent J, Nicolàs A (2009) Mediterranean Theileriosis and Other Tick Transmitted Piroplasmoses in Cattle in Minorca (Balearic Islands, Spain): the effect of tick control on prevalence levels analyzed by reverse line blot (Rlb) Macroarrays. J Parasitol 95:598–603. https://doi.org/10.1645/ge-1687.1
Kuibagarov M, Makhamed R, Zhylkibayev A, Berdikulov M, Abdrakhmanov S, Kozhabayev M, Akhmetollayev I, Mukanov K, Ryskeldina A, Ramankulov Y, Shustov A, Bauer C, Shevtsov A (2023) Theileria and Babesia infection in cattle – First molecular survey in Kazakhstan. Ticks Tick Borne Dis 14:102078. https://doi.org/10.1016/j.ttbdis.2022.102078
Elsify A, Sivakumar T, Nayel M, Salama A, Elkhtam A, Rizk M, Mosaab O, Sultan K, Elsayed S, Igarashi I, Yokoyama N (2015) An epidemiological survey of bovine Babesia and Theileria parasites in cattle, buffaloes, and sheep in Egypt. Parasitol Int 64:79–85. https://doi.org/10.1016/j.parint.2014.10.002
Callow LL, Mellors LT, McGregor W (1979) Reduction in virulence of Babesia bovis due to rapid passage in splenectomized cattle. Int J Parasitol 9:333–338. https://doi.org/10.1016/0020-7519(79)90083-3
Byaruhanga C, Makgabo SM, Choopa CN, Mulandane FC, Vorster I, Troskie M, Chaisi ME, Collins NE (2023) Genetic diversity in Babesia bovis from southern Africa and estimation of B. bovis infection levels in cattle using an optimised quantitative PCR assay. Ticks Tick Borne Dis 14:102084. https://doi.org/10.1016/j.ttbdis.2022.102084
Awad H, Antunes S, Galindo RC, do Rosário VE, de la Fuente J, Domingos A, El Hussein AM (2011) Prevalence and genetic diversity of Babesia and Anaplasma species in cattle in Sudan. Vet Parasitol 181:146–152. https://doi.org/10.1016/j.vetpar.2011.04.007
Taha KM, Salih DA, Ahmed BM, Enan KA, Ali AM, ElHussein AM (2011) First confirmed report of outbreak of malignant ovine theileriosis among goats in Sudan. Parasitol Res 109:1525–1527. https://doi.org/10.1007/s00436-011-2428-y
Rjeibi MR, Gharbi M, Mhadhbi M, Mabrouk W, Ayari B, Nasfi I, Jedidi M, Sassi L, Rekik M, Darghouth MA (2014) Prevalence of piroplasms in small ruminants in North-West Tunisia and the first genetic characterisation of Babesia ovis in Africa. Parasite 21:23. https://doi.org/10.1051/parasite/2014025
Hussein NM, Mohammed ES, Hassan AA, El-Dakhly KM (2017) Distribution pattern of Babesia and Theileria species in sheep in Qena Province, Upper Egypt. Arch Parasitol 1:1–4
Lee S-H, Mossaad E, Ibrahim AM, Ismail AA, Moumouni PFA, Liu M, Ringo AE, Gao Y, Guo H, Li J (2018) Detection and molecular characterization of tick-borne pathogens infecting sheep and goats in Blue Nile and West Kordofan states in Sudan. Ticks Tick Borne Dis 9:598–604. https://doi.org/10.1016/j.ttbdis.2018.01.014
Abdullah HHAM, Amanzougaghene N, Dahmana H, Louni M, Raoult D, Mediannikov O (2021) Multiple vector-borne pathogens of domestic animals in Egypt. PLoS Negl Trop Dis 15:e0009767. https://doi.org/10.1371/journal.pntd.0009767
Onyiche TE, MacLeod ET (2023) Hard ticks (Acari: Ixodidae) and tick-borne diseases of sheep and goats in Africa: A review. Ticks Tick Borne Dis 14:102232. https://doi.org/10.1016/j.ttbdis.2023.102232
OIE, (2008) Manual of Diagnostic Tests and Vaccines, Paris (2008) Bovine asbestosis. World Organ. Anim. Heal.
Martins TF, Onofrio VC, Barros-Battesti DM, Labruna MB (2010) Nymphs of the genus Amblyomma (Acari: Ixodidae) of Brazil: descriptions, redescriptions, and identification key. Ticks Tick Borne Dis 1:75–99. https://doi.org/10.1016/j.ttbdis.2010.03.002
Mtshali MS, Mtshali PS (2013) Molecular diagnosis and phylogenetic analysis of Babesia bigemina and Babesia bovis hemoparasites from cattle in South Africa. BMC Vet Res 9:1–7. https://doi.org/10.1186/1746-6148-9-154
Zeb J, Shams S, Din IU, Ayaz S, Khan A, Nasreen N, Khan H, Khan MA, Senbill H (2020) Molecular epidemiology and associated risk factors of Anaplasma marginale and Theileria annulata in cattle from North-western Pakistan. Vet Parasitol 279:109044. https://doi.org/10.1016/j.vetpar.2020.109044
Boussaadoun MA, Gharbi M, Sayeh L, Soudani MC, Darghouth MA (2015) Epidemiological Situation of Bovine Tropical Theileriosis (Theileria annulata Infection) in the Northwest Tunisia. J Adv Parasitol 2:69–74. https://doi.org/10.14737/journal.jap/2015/2.4.69.74
Al-Fahdi A, Alqamashoui B, Al-Hamidhi S, Kose O, Tageldin MH, Bobade P, Johnson EH, Hussain A-R, Karagenc T, Tait A (2017) Molecular surveillance of Theileria parasites of livestock in Oman. Ticks Tick Borne Dis 8:741–748. https://doi.org/10.1016/j.ttbdis.2017.05.008
Selim A, Attia K, AlKahtani MDF, Albohairy FM, Shoulah S (2022) Molecular epidemiology and genetic characterization of Theileria orientalis in cattle. Trop Anim Health Prod 54:178. https://doi.org/10.1007/s11250-022-03176-w
Ola-Fadunsin SD, Sharma RSK, Abdullah DA, Gimba FI, Jesse FFA, Sani RA (2020) Molecular detection, prevalence and risk factors of Theileria orientalis infection among cattle in Peninsular Malaysia. Prev Vet Med 180:105027. https://doi.org/10.1016/j.prevetmed.2020.105027
Marcellino WL, Salih DA, Julla II, El Hussein ARM (2012) Seroprevalence of East Coast fever in central equatoria state, South Sudan. Vet Ital 48:379–385
Glass EJ, Preston PM, Springbett A, Craigmile S, Kirvar E, Wilkie G, Brown CGD (2005) Bos taurus and Bos indicus (Sahiwal) calves respond differently to infection with Theileria annulata and produce markedly different levels of acute phase proteins. Int J Parasitol 35:337–347. https://doi.org/10.1016/j.ijpara.2004.12.006
El-Ashker M, Hotzel H, Gwida M, El-Beskawy M, Silaghi C, Tomaso H (2015) Molecular biological identification of Babesia, Theileria, and Anaplasma species in cattle in Egypt using PCR assays, gene sequence analysis and a novel DNA microarray. Vet Parasitol 207:329–334. https://doi.org/10.1016/j.vetpar.2014.12.025
Hailemariam Z, Krücken J, Baumann M, Ahmed JS, Clausen P-H, Nijhof AM (2017) Molecular detection of tick-borne pathogens in cattle from Southwestern Ethiopia. PLoS ONE 12:e0188248. https://doi.org/10.1371/journal.pone.0188248
Amira A-H, Răileanu C, Tauchmann O, Fischer S, Nijhof AM, Silaghi C (2021) Tick species identification and molecular detection of tick-borne pathogens in blood and ticks collected from cattle in Egypt. Ticks Tick Borne Dis 12:101676. https://doi.org/10.1016/j.ttbdis.2021.101676
Ganguly A, Maharana BR, Ganguly I (2020) Pentaplex PCR assay for rapid differential detection of Babesia bigemina, Theileria annulata, Anaplasma marginale and Trypanosoma evansi in cattle. Biologicals 63:81–88. https://doi.org/10.1016/j.biologicals.2019.10.011
Ziam H, Ababou A, Kazadi JM, Harhour KH, Assisi M, Geysen D, Berkvens D, (2016) Prévalences et signes cliniques associés des piroplasmoses bovines dans les Wilayates d’Annaba et El Tarf, Algérie.
Ayadi O, Rjeibi MR (2016) Prevalence and risk factors of tropical theileriosis, and sequencing of Theileria annulata, the causative pathogen, in Setif region (Algeria) before and after tick season. Rev d’élevage médecine vétérinaire des pays Trop 69:161–166
Foughali AA, Ziam H, Aiza A, Boulkrout H, Berber A, Bitam I, Gharbi M (2021) Cross-sectional survey of cattle haemopathogens in Constantine, Northeast Algeria. Vet Med Sci 7:1237–1244. https://doi.org/10.1002/vms3.459
Acknowledgements
We would like to thank all authors whose articles are included in this study.
Funding
No funding was received for the conduct of this study.
Author information
Authors and Affiliations
Contributions
AN designed the study, conducted literature research, collected, analyzed and interpreted data. AN wrote the original draft. MBS and AN edited and finalized the manuscript. All authors approved the final version of the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors have no conflict of interest to disclose.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Nahal, A., Ben Said, M. Systematic Review and Meta-Analysis on Piroplasma spp. Infection and Co-infection with Anaplasma marginale in Domestic Ruminants from Algeria. Acta Parasit. 69, 135–151 (2024). https://doi.org/10.1007/s11686-023-00768-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11686-023-00768-w