Abstract
Purpose
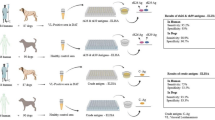
Visceral leishmaniasis (VL) is a systemic and parasitic disease that is usually fatal if left untreated. VL is endemic in different parts of Iran and is caused mainly by Leishmania infantum. This study aimed to recognition immunoreactive proteins in amastigote-like and promastigote stages of L. infantum (Iranian strain) by antibodies present in the sera of VL patients.
Methods
Total protein extract from amastigote-like and promastigote cells was separated by two-dimensional electrophoresis (2DE). To detect the immunoreactive proteins, 2DE immunoblotting method was performed using different pools of VL patients’ sera.
Results
Approximately 390 and 430 protein spots could be separated in 2DE profiles of L. infantum amastigote-like and promastigote stages, respectively. In immunoblotting method, approximately 295 and 135 immunoreactive proteins of amastigotes-like reacted with high antibody titer serum pool and low antibody titer serum pool, respectively. Approximately 120 and 85 immunoreactive proteins of promastigote extract were recognized using the high antibody titer sera pool and low antibody titer sera, respectively.
Conclusion
The present study has recognized a number of antigenic diversity proteins based on the molecular weight and pH in amastigote-like and promastigote stages of L. infantum. These results provide us a new concept for further analysis development in the field of diagnosis biomarkers and vaccine targets.


Similar content being viewed by others
Data availability
The datasets analysed during the current study are available from the corresponding author on reasonable request.
References
World Health Organization. Leishmaniasis. Available from: https://www.who.int/news-room/fact-sheets/detail/leishmaniasis. [Accessed: 2021-1-24].
Mohebali M (2013) Visceral leishmaniasis in Iran: review of the epidemiological and clinical features. Iran J Parasitol 8:348–358
Hajjaran H, Saberi R, Borjian A, Fakhar M, Hosseini SA, Ghodrati S et al (2021) The geographical distribution of human cutaneous and visceral leishmania species identified by molecular methods in Iran: a systematic review with meta-analysis. Front Public Health 9:661674. https://doi.org/10.3389/fpubh.2021.661674
Elmahallawy EK, Martínez AS, Rodriguez-Granger J, Hoyos-Mallecot Y, Agil A, Mari JMN et al (2014) Diagnosis of leishmaniasis. J Infect Dev Ctries 8(08):961–972. https://doi.org/10.3855/jidc.4310
Chappuis F, Sundar S, Hailu A, Ghalib H, Rijal S, Peeling RW et al (2007) Visceral leishmaniasis: what are the needs for diagnosis, treatment and control? Nat Rev Microbiol 5(11):873–882. https://doi.org/10.1038/nrmicro1748
Torres-Guerrero E, Quintanilla-Cedillo MR, Ruiz-Esmenjaud J, Arenas R (2017) Leishmaniasis: a review. F1000Research 6:750. https://doi.org/10.12688/f1000research.11120.1
Zilberstein D, Shapira M (1994) The role of pH and temperature in the development of Leishmania parasites. Annu Rev Microbiol 48:449–470. https://doi.org/10.1146/annurev.mi.48.100194.002313
Alexander J, Russell DG (1992) The interaction of Leishmania species with macrophages. Adv Parasitol 31:175–254. https://doi.org/10.1016/S0065-308X(08)60022-6
Matlashewski G (2001) Leishmania infection and virulence. Med Microbiol Immunol 190(1):37–42. https://doi.org/10.1007/s004300100076
Saar Y, Ransford A, Waldman E, Mazareb S, Amin-Spector S, Plumblee J et al (1998) Characterization of developmentally-regulated activities in axenic amastigotes of Leishmania donovani. Mol Biochem Parasitol 95(1):9–20. https://doi.org/10.1016/S0166-6851(98)00062-0
Debrabant A, Joshi MB, Pimenta PF, Dwyer DM (2004) Generation of Leishmania donovani axenic amastigotes: their growth and biological characteristics. Int J Parasitol 34(2):205–217. https://doi.org/10.1016/j.ijpara.2003.10.011
Thiel M, Bruchhaus I (2001) Comparative proteome analysis of Leishmania donovani at different stages of transformation from promastigotes to amastigotes. Med Microbiol Immunol 190(1):33–36. https://doi.org/10.1007/s004300100075
Biyani N, Madhubala R (2012) Quantitative proteomic profiling of the promastigotes and the intracellular amastigotes of Leishmania donovani isolates identifies novel proteins having a role in Leishmania differentiation and intracellular survival. Biochim et Biophys Acta (BBA) Proteins Proteom. 1824(12):1342–1350. https://doi.org/10.1016/j.bbapap.2012.07.010
Rashidi S, Mojtahedi Z, Shahriari B, Kalantar K, Ghalamfarsa G, Mohebali M (2019) An immunoproteomic approach to identifying immunoreactive proteins in Leishmania infantum amastigotes using sera of dogs infected with canine visceral leishmaniasis. Pathog Glob Health 113(3):124–132. https://doi.org/10.1080/20477724.2019.1616952
Lage DP, Ludolf F, Silveira PC, Machado AS, Ramos FF, Dias DS et al (2019) Screening diagnostic candidates from Leishmania infantum proteins for human visceral leishmaniasis using an immunoproteomics approach. Parasitology 146(11):1467–1476. https://doi.org/10.1017/S0031182019000714
Coelho VT, Oliveira JS, Valadares DG, Chavez-Fumagalli MA, Duarte MC, Lage PS et al (2012) Identification of proteins in promastigote and amastigote-like Leishmania using an immunoproteomic approach. PLoS Negl Trop Dis 6(1):e1430. https://doi.org/10.1371/journal.pntd.0001430
Damerval C, De Vienne D, Zivy M, Thiellement H (1986) Technical improvements in two-dimensional electrophoresis increase the level of genetic variation detected in wheat-seedling proteins. Electrophoresis 7(1):52–54. https://doi.org/10.1002/elps.1150070108
Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72(1–2):248–254. https://doi.org/10.1016/0003-2697(76)90527-3
Kumari S, Kumar A, Samant M, Sundar S, Singh N, Dube A (2008) Proteomic approaches for discovery of new targets for vaccine and therapeutics against visceral leishmaniasis. Proteomics Clin Appl 2(3):372–386. https://doi.org/10.1002/prca.200780017
Ejazi SA, Bhattacharyya A, Choudhury ST, Ghosh S, Sabur A, Pandey K et al (2018) Immunoproteomic identification and characterization of Leishmania membrane proteins as non-invasive diagnostic candidates for clinical visceral leishmaniasis. Sci Rep 8(1):1–11. https://doi.org/10.1038/s41598-018-30546-y
Kumari S, Samant M, Misra P, Khare P, Sisodia B, Shasany AK et al (2008) Th1-stimulatory polyproteins of soluble Leishmania donovani promastigotes ranging from 89.9 to 97.1 kDa offers long-lasting protection against experimental visceral leishmaniasis. Vaccine 26(45):5700–5711. https://doi.org/10.1016/j.vaccine.2008.08.021
Dea-Ayuela MA, Rama-Iñiguez S, Bolás-Fernández F (2006) Proteomic analysis of antigens from Leishmania infantum promastigotes. Proteomics 6(14):4187–4194. https://doi.org/10.1002/pmic.200600101
Sharma S, Singh G, Chavan HD, Dey CS (2009) Proteomic analysis of wild type and arsenite-resistant Leishmania donovani. Exp Parasitol 123(4):369–376. https://doi.org/10.1016/j.exppara.2009.08.003
Chambers G, Lawrie L, Cash P, Murray GI (2000) Proteomics: a new approach to the study of disease. J Pathol 192(3):280–288. https://doi.org/10.1002/1096-9896(200011)192:3%3c280::AID-PATH748%3e3.0.CO;2-L
Heidari S, Hajjaran H, Kazemi B, Gharechahi J, Mohebali M, Ranjbar MM et al (2021) Identification of immunodominant proteins of Leishmania infantum by immunoproteomics to evaluate a recombinant multi-epitope designed antigen for serodiagnosis of human visceral leishmaniasis. Exp Parasitol 222:108065. https://doi.org/10.1016/j.exppara.2021.108065
Mukherjee I, Chakraborty A, Chakrabarti S (2016) Identification of internalin-A-like virulent proteins in Leishmania donovani. Parasit Vectors 9(1):1–17. https://doi.org/10.1186/s13071-016-1842-5
Jain R, Ghoshal A, Mandal C, Shaha C (2010) Leishmania cell surface prohibitin: role in host–parasite interaction. Cell Microbiol 12(4):432–452. https://doi.org/10.1111/j.1462-5822.2009.01406.x
Chávez-Fumagalli MA, Schneider MS, Lage DP, Machado-de-Ávila RA, Coelho EA (2017) An in silico functional annotation and screening of potential drug targets derived from Leishmania spp. hypothetical proteins identified by immunoproteomics. Exp Parasitol 176:66–74. https://doi.org/10.1016/j.exppara.2017.03.005
Davis AJ, Perugini MA, Smith BJ, Stewart JD, Ilg T, Hodder AN et al (2004) Properties of GDP-mannose pyrophosphorylase, a critical enzyme and drug target in Leishmania mexicana. J Biol Chem 279(13):12462–12468. https://doi.org/10.1074/jbc.M312365200
Maspi N, Ghaffarifar F, Sharifi Z, Dalimi A (2015) Cloning and constructing a plasmid encoding Leishmania eukaryotic initiation factor gene of Leishmania major fused with green fluorescent protein gene as a vaccine candidate. West Indian Med J 65:256–259. https://doi.org/10.7727/wimj.2014.201
Castro H, Romao S, Gadelha FR, Tomás AM (2008) Leishmania infantum: provision of reducing equivalents to the mitochondrial tryparedoxin/tryparedoxin peroxidase system. Exp parasitol 120(4):421–423. https://doi.org/10.1016/j.exppara.2008.09.002
Zhang WW, Charest H, Ghedin E, Matlashewski G (1996) Identification and overexpression of the A2 amastigote-specific protein in Leishmania donovani. Mol Biochem Parasitol 78(1–2):79–90. https://doi.org/10.1016/S0166-6851(96)02612-6
Alcolea PJ, Alonso A, Gómez MJ, Postigo M, Molina R, Jiménez M (2014) Stage-specific differential gene expression in Leishmania infantum: from the foregut of Phlebotomus perniciosus to the human phagocyte. BMC Genom 15(1):1–16. https://doi.org/10.1186/1471-2164-15-849
Drini S, Criscuolo A, Lechat P, Imamura H, Skalickỳ T, Rachidi N et al (2016) Species-and strain-specific adaptation of the HSP70 super family in pathogenic trypanosomatids. Genome Biol Evol 8(6):1980–1995. https://doi.org/10.1093/gbe/evw140
Almeida MS, Pereira BAS, Guimarães MLR, Alves CR (2012) Proteinases as virulence factors in Leishmania spp. infection in mammals. Parasit Vectors 5(160):10. https://doi.org/10.1186/1756-3305-5-160
Santos CX, Stolf BS, Takemoto PV, Amanso AM, Lopes LR, Souza EB et al (2009) Protein disulfide isomerase (PDI) associates with NADPH oxidase and is required for phagocytosis of Leishmania chagasi promastigotes by macrophages. J Leukoc Biol 86(4):989–998. https://doi.org/10.1189/jlb.0608354
Dey R, Meneses C, Salotra P, Kamhawi S, Nakhasi HL, Duncan R (2010) Characterization of a Leishmania stage-specific mitochondrial membrane protein that enhances the activity of cytochrome c oxidase and its role in virulence. Mol Microbiol 77(2):399–414. https://doi.org/10.1111/j.1365-2958.2010.07214.x
Ball WB, Kar S, Mukherjee M, Chande AG, Mukhopadhyaya R, Das PK (2011) Uncoupling protein 2 negatively regulates mitochondrial reactive oxygen species generation and induces phosphatase-mediated anti-inflammatory response in experimental visceral leishmaniasis. J Immunol 187(3):1322–1332. https://doi.org/10.4049/jimmunol.1004237
Yurchenko V, Xue Z, Sherry B, Bukrinsky M (2008) Functional analysis of Leishmania major cyclophilin. Int J Parasitol 38(6):633–639. https://doi.org/10.1016/j.ijpara.2007.10.001
Marcelain K, Colombo A, Molina MC, Ferreira L, Lorca M, Aguillón JC et al (2000) Development of an immunoenzymatic assay for the detection of human antibodies against Trypanosoma cruzi calreticulin, an immunodominant antigen. Acta Trop 75(3):291–300. https://doi.org/10.1016/S0001-706X(00)00062-0
Jensen AT, Ismail A, Gaafar A, El Hassan AM, Theander TG (2002) Humoral and cellular immune responses to glucose regulated protein 78–a novel Leishmania donovani antigen. Trop Med Int Health 7(5):471–476. https://doi.org/10.1046/j.1365-3156.2002.00880.x
Trujillo C, Ramı́rez R, Vélez ID, Berberich C, (2000) The humoral immune response to the kinetoplastid membrane protein-11 in patients with American leishmaniasis and Chagas disease: prevalence of IgG subclasses and mapping of epitopes. Immunol Lett 70(3):203–209. https://doi.org/10.1016/S0165-2478(99)00146-7
Pateraki E, Portocala R, Labrousse H, Guesdon JL (1983) Antiactin and antitubulin antibodies in canine visceral leishmaniasis. Infect Immun 42(2):496–500. https://doi.org/10.1128/iai.42.2.496-500.1983
Duarte MC, Lage DP, Martins VT, Costa LE, Salles BCS, Carvalho AMRS et al (2017) Performance of Leishmania braziliensis enolase protein for the serodiagnosis of canine and human visceral leishmaniosis. Vet Parasitol 238:77–81. https://doi.org/10.1016/j.vetpar.2017.03.024
Santarém N, Tomás A, Ouaissi A, Tavares J, Ferreira N, Manso A et al (2005) Antibodies against a Leishmania infantum peroxiredoxin as a possible marker for diagnosis of visceral leishmaniasis and for monitoring the efficacy of treatment. Immunol Lett 101(1):18–23. https://doi.org/10.1016/j.imlet.2005.04.006
Oliveira-da-Silva JA, Machado AS, Ramos FF, Tavares GS, Lage DP, Ludolf F et al (2020) Evaluation of Leishmania infantum pyridoxal kinase protein for the diagnosis of human and canine visceral leishmaniasis. Immunol Lett 220:11–20. https://doi.org/10.1016/j.imlet.2020.01.005
Santos TT, Cardoso MS, Machado AS, Siqueira WF, Ramos FF, Oliveira-da-Silva JA et al (2019) Recombinant Leishmania eukaryotic elongation factor-1 beta protein: a potential diagnostic antigen to detect tegumentary and visceral leishmaniasis in dogs and humans. Microb Pathog 137:103783. https://doi.org/10.1016/j.micpath.2019.103783
Sánchez-Cañete MP, Carvalho L, Pérez-Victoria FJ, Gamarro F, Castanys S (2009) Low plasma membrane expression of the miltefosine transport complex renders Leishmania braziliensis refractory to the drug. Antimicrob Agents Chemother 53(4):1305–1313. https://doi.org/10.1128/AAC.01694-08
Nandan D, Thomas SA, Nguyen A, Moon KM, Foster LJ, Reiner NE (2017) Comprehensive identification of mRNA-binding proteins of Leishmania donovani by interactome capture. PLoS ONE 12(1):e0170068. https://doi.org/10.1371/journal.pone.0170068
Ishemgulova A, Kraeva N, Hlaváčová J, Zimmer SL, Butenko A, Podešvová L et al (2017) A putative ATP/GTP binding protein affects Leishmania mexicana growth in insect vectors and vertebrate hosts. PLOS Negl Trop Dis 11(7):e0005782. https://doi.org/10.1371/journal.pntd.0005782
Naula C, Parsons M, Mottram JC (2005) Protein kinases as drug targets in trypanosomes and Leishmania. Biochim Biophys Acta Proteins Proteom 1754(1–2):151–159. https://doi.org/10.1016/j.bbapap.2005.08.018
Carrillo E, Crusat M, Nieto J, Chicharro C, del Carmen TM, Martínez E et al (2008) Immunogenicity of HSP-70, KMP-11 and PFR-2 leishmanial antigens in the experimental model of canine visceral leishmaniasis. Vaccine 26(15):1902–1911. https://doi.org/10.1016/j.vaccine.2008.01.042
Streit JA, Recker TJ, Donelson JE, Wilson ME (2000) BCG expressing LCR1 of Leishmania chagasi induces protective immunity in susceptible mice. Exp Parasitol 94(1):33–41. https://doi.org/10.1006/expr.1999.4459
Chávez-Fumagalli MA, Costa MA, Oliveira DM, Ramírez L, Costa LE, Duarte MC et al (2010) Vaccination with the Leishmania infantum ribosomal proteins induces protection in BALB/c mice against Leishmania chagasi and Leishmania amazonensis challenge. Microbes Infect 12(12–13):967–977. https://doi.org/10.1016/j.micinf.2010.06.008
Yousif NM, Musa AM, Elhassan AM, Khalil EA, Elhassan IM (2007) Anti-glucose regulated protein 78 (GRP78) antibody responses in Alum/ALM plus BCG vaccinee, visceral leishmaniasis, and Post Kala-azar Dermal leishmaniasis patients. Sudan J Med Sci 2(3):175–179. https://doi.org/10.4314/sjms.v2i3.38484
Basu R, Bhaumik S, Basu JM, Naskar K, De T, Roy S (2005) Kinetoplastid membrane protein-11 DNA vaccination induces complete protection against both pentavalent antimonial-sensitive and-resistant strains of Leishmania donovani that correlates with inducible nitric oxide synthase activity and IL-4 generation: evidence for mixed Th1-and Th2-like responses in visceral leishmaniasis. J Immunol 174(11):7160–7171. https://doi.org/10.4049/jimmunol.174.11.7160
Aguilar-Be I, da Silva ZR, Paraguai de Souza E, Borja-Cabrera GP, Rosado-Vallado M, Mut-Martin M et al (2005) Cross-protective efficacy of a prophylactic Leishmania donovani DNA vaccine against visceral and cutaneous murine leishmaniasis. Infect Immun 73(2):812–819. https://doi.org/10.1128/IAI.73.2.812-819.2005
Acknowledgements
This work was supported by the National Institute for Medical Research Development (NIMAD) (Grant No. 958753).
Author information
Authors and Affiliations
Contributions
SH: investigation, formal analysis, visualization, and writing—original draft; HH: conceptualization, supervision, project administration, funding acquisition, validation, and resources; MM: editing of manuscript; BA: resources and investigation; JG: editing of manuscript and English correction of the final draft.
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflict of interest.
Ethical Approval
This study was approved by the Ethics Committee of Tehran University of Medical Sciences. (Ethics Approval No. IR-TUMS-VCR-REC-1395–721).
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Heidari, S., Hajjaran, H., Mohebali, M. et al. Recognition of Immunoreactive Proteins in Leishmania infantum Amastigote-Like and Promastigote Using Sera of Visceral Leishmaniasis Patients: a Preliminary Study. Acta Parasit. 69, 533–540 (2024). https://doi.org/10.1007/s11686-023-00764-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11686-023-00764-0