Abstract
Mediterranean type of visceral leishmaniasis (VL) is a zoonotic parasitic infection. Some provinces of Iran are endemic for VL while other parts are considered as sporadic areas. This study aimed to assess a combination of recombinant K26 and rK39 antigens as well as crude antigen (CA), derived from an Iranian strain of L. infantum, compared to direct agglutination test (DAT) for the detection of VL in humans and domestic dogs as animal reservoir hosts of the disease. A combination of rK26 and rK39 antigens and also CA was evaluated using indirect ELISA on serum samples of 171 VL confirmed humans (n = 84) and domestic dogs (n = 87) as well as 176 healthy humans (n = 86) and domestic dogs (n = 90). Moreover, 36 serum samples of humans (n = 20) and canines (n = 16) with other potentially infectious diseases were collected and tested for finding cross- reactivity. The results of ELISA were compared to DAT, currently considered as gold standard for the serodiagnosis of VL. The sensitivity and specificity, positive predictive and negative predictive values were calculated compared to DAT. The positive sera had previously shown a positive DAT titer ≥ 1:800 for humans and ≥ 1:80 for dogs. Analysis was done by MedCalc and SPSS softwares. Using the combination of rK26 and rK39 in ELISA, a sensitivity of 95.2% and a specificity of 93.0% % were found in human sera at a 1:800 (cut-off) titer when DAT-confirmed cases were compared with healthy controls; a sensitivity of 98.9% and specificity of 96.7%% were found at a 1:80 (cut-off) titer compared with DAT. A good degree of agreement was found between the combined rK39 and rK26-ELISA with DAT in human (0.882) and dog serum samples (0.955) by kappa analysis (p < 0.05). The ELISA using the CA test showed 75% sensitivity in human and 93.1% in dog serum samples as well as 53.5% specificity in human and 83.3% in dog,s sera, respectively. The combination of rK26 and rK39 recombinant antigen prepared from Iranian strain of Leishmania infantum showed high accuracy for the serodiagnosis of VL in human and domestic dogs. Further extended field trial with a larger sample size is recommended.
Similar content being viewed by others
Introduction
Visceral leishmaniasis (VL), is a systemic parasitic disease caused by protozoa of the Leishmania donovani complex. According to global health statistics, VL remains one of the more serious parasitic diseases with outbreaks and mortality potential. An estimated 50,000 to 90,000 new cases of VL occur worldwide annually. More than 90% of new visceral leishmaniasis (VL) cases were reported from 10 countries including Brazil, China, Ethiopia, India, Iraq, Kenya, Nepal, Somalia, South Sudan and Sudan in 20201.
Mediterranean visceral leishmaniasis (MVL) caused by Leishmania infantum has been reported in northwest, southwest, southern, and northeastern parts of Iran2, 3. In VL-endemic areas of Iran, 90% of 1698 serologically positive VL cases were children younger than 12 years during 2002 to 20123.
Dogs are considered to be a reservoir for L. infantum and have a main role in zoonotic transmission of MVL4. However, asymptomatic humans can also be responsible for anthroponotic transmission5. Then, early diagnosis of infection in dogs and humans is necessary in order to reduce the number of VL cases in endemic areas6.
Although, parasitological methods have 100% specificity for the diagnosis of VL, their sensitivity is relatively low and these methods are invasive7. Serological tests, based on antibody detection, such as direct agglutination test (DAT), immunofluorescence assay (IFA), an enzyme-linked immunosorbent assay (ELISA) are available. DAT is an appropriate tool for the serodiagnosis of human VL with high sensitivity and specificity7. As DAT is a simple, accurate and efficient serological test, it was recommended for serodiagnosis of human VL (HVL) as well as canine VL (CVL) particularly in endemic areas8.
Kinesin-related conserved recombinant antigens (i.e. rK39, rK26, rK9, rK28, rKLO8, rKRP42) have been tested for improved sensitivity and specificity in the detection of antibodies against VL9, 10. Moreover, the immunochromatographic tests (ICT) based on these antigens are very useful, low-cost, rapid, and practical in the field. ELISA tests based on rK39 and rK26 recombinant antigens have shown acceptable sensitivity and specificity in the studies performed on human and dog sera in different areas11, 12. Our previous investigation demonstrated that the recombinant K39 antigen from an Iranian strain of L. infantum has high sensitivity and specificity in symptomatic HVL and CVL13. Therefore, we decided to determine the sensitivity and specificity of a combination of rK39 and rK26 Ag prepared from an Iranian strain of L. infantum (MCAN/IR/14/M14 with GenBank Accession number KT201383) to detect anti-Leishmania infantum antibodies in both symptomatic and asymptomatic human and domestic dogs.
Results
Assessment of L. infantum mixed rK39 and rK26 recombinant antigen compared to DAT on human and domestic dog serum samples using ELISA
Altogether, 84 DAT positive sera from VL- infected humans with anti-Leishmania antibodies at ≥ 1:800, 87 DAT positive sera from VL- infected dogs with anti-Leishmania antibodies at ≥ 1:80 and 176 sera from healthy controls (86 human and 90 dog serum samples) were tested by the combination of L. infantum rK39 and rK26 and CA, respectively. It is necessary to mention that 36 serum samples from patients with other potentially cross-reactive infectious diseases including 20 infected human sera and 16 infected dog sera were assayed in the control group (Tables 1 and 2). In this study, 25 of 52 symptomatic humans with a DAT titer ≥ 1:3200 were also checked by parasitology methods (microscopy and culture).
Data analysis indicated that the total sensitivity and specificity of the rK39 and rK26 antigens used as a mixture, were 95.2% and 93% in humans and 98.9% and 96.7% in dogs, respectively; these values were lower in CA-ELISA with 75% and 52.3% in humans, 93.1% and 83.3% in dogs, respectively (Table 3). The sensitivity of the mixed rK39 and rK26-ELISA for sera from symptomatic (≥ 1:3200) (n = 52) and asymptomatic (≤ 1:1600) (n = 32) humans was 100% and 87.5%, respectively, with a 93% specificity. CA-ELISA showed a sensitivity of 85.2% for symptomatic individuals, whereas it had a sensitivity of approximately 59.4% for asymptomatic individuals. The CA-ELISA had a specificity of 53.5% in humans with high DAT titers (≥ 1:3200), but only 52.3% in humans with lower DAT titers (≤ 1:1600) (Table 4).
McNemar's test revealed no significant differences between mixed rK39 and rK26—ELISA and DAT results in humans P = 0.745 and dogs P = 0. 625. For human samples, there was a significant difference when comparing the CA-ELISA to the DAT (P = 0.02), but not for dogs (P = 0.078).
The k index was used to assess the amount of agreement between mixed rK39 and rK26 and CA-ELISA and DAT using SPSS software. In asymptomatic humans, there was complete agreement between mixed rK39 and rK26 -ELISA and DAT (k = 0.790), but no concordance of CA-ELISA with DAT (k = 0.092); in symptomatic humans, the degree of agreement was very high using the mixed rK39 and rK26 ELISA (k = 0.909), but weak in CA-ELISA (k = 0.340).
The k coefficient for mixed rK39 and rK26 ELISA and CA-ELISA in humans was 0.882 and 0.284, respectively. In dogs, these agreement values revealed nearly perfect agreement for mixed rK39 and rK26, as well as a substantial result for CA with DAT (k = 0.995 for mixed rK39 and rK26—ELISA and k = 0.763 for CA-ELISA) (Table 5).
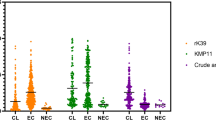
The Youden's indexes for mixed rK39 and rK26 -ELISA and CA-ELISA were 0.882 and 0.285 in human reservoirs, respectively, and 0.956 and 0.764 in dog reservoirs, indicating that mixed rK39 and rK26 -ELISA has a high accuracy in human and canine reservoirs, while CA-ELISA has an acceptable accuracy in dog (Fig. 1).
Cross-reactivity with the sera from individuals with other infectious diseases was not seen in the mixed rK39 and rK26 ELISA either in dogs or in humans. Six sera of healthy people were positive and reacted with the mixed antigens. Cross-reactivity of VL-negative human sera infected by cutaneous leishmaniasis (n = 4) and tuberculosis (n = 4) were seen for CA in this study.
Discussion
The sensitivity and specificity of the diagnostic tests currently employed in Iran to assess CVL in asymptomatic and symptomatic VL in the field have been challenged, which has had a negative impact on the effectiveness of control measures14. Many endemic regions have heterogeneity of Leishmania parasites. In a particular population, tests based on homologous Leishmania antigens are required to detect cases that are difficult to diagnose with currently available tests15.
The current study shows that combining rK39 and rK26 from an Iranian strain of L. infantum in a single-well test has a high sensitivity and specificity for VL diagnosis in both symptomatic and asymptomatic reservoirs. This test proved to be superior to CA ELISA in terms of specificity, sensitivity and agreement with DAT.
This study builds on our previous results where we had already shown the recombinant rK39 antigen from an Iranian strain of L. infantum (MCAN/IR/14/M14 with GenBank Accession number KT201383) showed a high sensitivity and specificity at identifying human and dog cases with symptomatic VL13.
rK39 antigen is a 39-amino-acid repetitive immunodominant B-cell epitope of the 230 kDa kinesin related protein of L. chagasi and rK26 hydrophilic antigens has 11 copies of a 14-amino-acid repeat16. Recently, Farajnia et al. had described a high sensitivity (96.8%) and specificity (100%) when using rK26 produced from an Iranian strain of L. infantum in VL serodiagnosis17. However, when rK26 from L. donovani strain was examined in the Indian subcontinent, the sensitivity of the test dropped to only 38%, while in Brazil, only 56% of cases of VL were found to be positive16, 18.
Dogs with clinical signs of leishmaniasis are the main reservoir of L. infantum for humans.
In areas where zoonotic VL is endemic, the prevalence of L. infantum in dogs is often high while many of them are asymptomatic. Domestic dogs infected by VL play an important role in transmission of VL to humans19. The control of VL depends on early case detection in humans and adequate treatment. Diagnostic tests play a major role in the control of VL, as well as patient management, screening of infections, and epidemiological studies20. Since some evidence is available on the great sensitivity of the rK26 antigen in the diagnosis of VL at the early stage of infection, we decided to mix the rK39 antigen with rK26 derived the same Iranian strain of L. infantum to diagnose VL in human and dog21, 22.
We showed that rK39 and the rK26 carry immunodominant epitopes which can be useful for the serological diagnosis of canine and human visceral leishmaniasis.
The antibody reactivity and sensitivity of these two recombinant antigens were high when they were employed in parallel, suggesting that combining them in a single-well test could improve the assay's performance even more23.
The results of this study demonstrated that a mixture of rK39 and rK26 ELISA could efficiently detect antibodies in infected cases achieving a high sensitivity of 95.2% for human sera and 98.9% for dog sera. A study in Brazil reported a similar sensitivity (100%) using rK39 and rK26 antigens by RDT (TRALd) in symptomatic CVL diagnosis24.
In present study, mixed rK39 and rK26 ELISA has shown approximately 100% sensitivity in cases of parasitologically confirmed VL and high antibody-titer in dogs and humans similarly to what seen in the rk39 ELISA13. There was statistically significant correlation between the antibody titer and sensitivity25,26,27,28. There are a variety of factors affecting the levels of antibody production, including the load and species of infecting parasite, the form of the disease, the genetic background of the host and vector-derived products29. Sex and age are additional factors that might affect the antibody response, but this was beyond the scope of this study.
Moreover, a meta-analysis of published data revealed that the rK39 RDT has a lower sensitivity in dogs with clinical infection (87%) than in symptomatic humans (94%) infected with L. donovani or L. infantum27. Our data indicated mixed rK39 and rK26 increases the sensitivity of the test in symptomatic CVL (98.9%). It is possible that this is due to the increase of rK39 and rK26 immunodominant epitopes in this ELISA test and more ability of anti-Leishmania antibodies in reservoirs to bind them.
The results of rK39/rK26 ELISA showed a high sensitivity rate of 87.5% in asymptomatic HVL. Low sensitivity of rK39-ELISA (62.5%) has been reported in self–healing or sub-clinical human infection in our previous study13.Other studies on asymptomatic HVL described similar sensitivity 62.5% using rK39-ELISA30, 31. A significant 25% increase in diagnostic sensitivity in the multiple epitopes format (rK39/rK26) ELISA was seen for asymptomatic cases, indicating the effectiveness of adding the rK26 antigen to rK39 in a single well.
Although the level of antibody titer is directly related to the tissue parasitic load and positivity rate in serological test, the results of the current study indicated that mixture of rK39 and rK26 increase the sensitivity of diagnosis in subclinical forms. In present study, there were only 3 dogs without clinical signs and all of them had a positive result for mixed rK39 and rK26 ELISA.
The k indexes between the rK39/rK26 ELISA and DAT were in complete agreement for the asymptomatic group (k = 0.790) and in perfect accordance in the human symptomatic group (k = 0.909) and dog reservoir (k = 0.995).
Although, lower sensitivity was reported for rK39/ rK26 ELISA in asymptomatic human cases than in symptomatic human ones in this study, all negative samples in the asymptomatic group were related to the cut-off antibody titer (1:800) and all asymptomatic human cases with anti-body titer 1:1600 have been diagnosed as VL positive.
In the case of the canine reservoirs, only three cases were asymptomatic, so a separate analysis was not performed for the asymptomatic group, but the three dogs with antibody titers of 1:160 were positive.
In patients with clinical symptoms, CA-ELISA had a sensitivity of 93.1% for CVL and 84.6% for HVL, respectively, but lower positivity rates of almost 59.4% were detected in sera with lower levels of antibodies (≤ 1:1600). Porrozzi et al. also reported a low CA-ELISA sensitivity (30%) in this group25. CA-ELISA has a higher sensitivity in symptomatic canines when compared to the same situation in human.
Surprisingly, despite a significant increase in sensitivity (84.6%) for CA-ELISA in HVL with clinical signs as compared to another group, it fails to achieve a good agreement in the connection with DAT (k = 0.340). Conversely, this value is acceptable in dogs with a high antibody titer (k = 0.763).
This study shows a 93% specificity for mixed rK39 and rK26 Ag in human, while the test specificity in the previous study, which was used rK39 Ag alone in the ELISA diagnostic wells, was 86%13. This 7% rise in specificity could be the effect of adding rK26 Ag and the increase in specific epitopes for detection of antibodies against L. infantum. So, it indicates the possibility of less cross-reactivity with the serum of healthy individuals.
Our results indicate the lower specificity (93%) of ELISA using mixed rK39 and rK26 Ag in human in comparison with specificity reported from Brazil (98%)24.This value was determined 96.7% when mixed rK39 and rK26 -ELISA was used to detect antibody in the canines. Boario et al. stated a 99% specificity in both human and canine control groups using Chimeric (K9, K26, and K39 antigens) ELISA32. However, cross-reactivity was not seen for mixed rK39 and rK26 -ELISA in human and dog sera with other infectious disease sera applied in the current study, the less specificity of mixed rK39 and rK26 has been reported in comparison with other researches. It is likely due to higher prevalence of other infectious diseases with similar signs.
None of the sera from individuals with other infectious diseases produced a positive reaction in the mixed rK39 and rK26 ELISA, while cross-reactivity of VL-negative sera infected by cutaneous leishmaniasis and tuberculosis were seen for CA in this study. Therefore, in contrast to CA-ELISA, the mixed rK39 and rK26 -ELISA can discriminate true leishmanial infection with more specificity.
Furthermore, when compared to combined rK39 and rK26, CA has a lower specificity (53.5% in humans and 83.3% in dogs). Reactions with healthy blood donors' sera and cross-reactivity with other infections could be the main33.
Overall, the high validation of the mixed rK39 and rK26 antigens comparable to DAT suggests that combination rK39 and rK26 seems to be a good option for diagnosing symptomatic and asymptomatic VL infected humans and dogs. CA-ELISA has a poor agreement with the gold standard DAT in humans, but an outstanding agreement with DAT in dogs.
Future efforts to optimize the binding of mixed rK39 and rK26 antigens to nitrocellulose strips could result in a speedy and cost-effective dipstick test that would change VL diagnosis in Iran and other countries where L. infantum is the predominant causative agent. Furthermore, this test improves the sensitivity of the assays for diagnosing VL in the asymptomatic population in Iran.
Methods
Ethics approval and consent to participate
This study was approved by the Ethical Committee of Tehran University of Medical Sciences (IR.TUMS.SPH.REC.1397.203 and 92-03-162-24558) in accordance with the Helsinki Declaration and guidelines. Dog sera were collected in coordination with Iran Veterinary Organization after informed consent from the owners. The human sera were collected from volunteers following informed consent. Children were included in this study after consent from their legal guardians.
Study population
The sample size was calculated based on the sensitivity and specificity of 70% obtained from studies done on rK39 dipstick test for diagnosis of VL in Iran34. The 95% confidence level with a margin of error of less than 10% was considered for this sample size.
Totally, 3 ml of 171 confirmed VL- infected sera with a positive DAT were collected between April 2016 and March 2018, using a non-probability convenience sampling method by Iranian Reference Laboratory for leishmaniasis in TUMS. The sera were kept frozen at − 20 °C after doing DAT until use in ELISA tests. All the serum samples including 84 humans and 87 dogs showed anti-Leishmania antibodies in DAT at ≥ 1:800 for humans and at ≥ 1:80 for dogs35.
Amastigotes were observed in bone marrow samples of 25 of 52 patients with active VL (with anti-Leishmania antibodies at ≥ 1:3200 titers); the remaining symptomatic patients (27 patients) showed clinical signs of VL including hepatosplenomegaly, anemia, and a prolonged irregular fever (n = 27). The number of asymptomatic patient sera with titer of anti-Leishmania antibodies less or equal to 1:1600 were 32.
Fifty-four serum samples were collected from dogs with pathognomonic clinical signs of VL including skin lesions, alopecia, diarrhea, and splenomegaly (without biopsy, n = 54). Amastigote forms were demonstrated in spleen and liver biopsy samples of 30 dogs with active VL (34.5%). All the collected sera from dogs with active VL showed anti-Leishmania antibody titers ≤ 1:320. Three dogs had parasitological positive biopsy samples with 1:160 anti-Leishmania antibody titers using DAT (n = 3).
In parallel, 3 ml serum sample were collected from 176 sera samples from healthy people in geographical areas non-endemic for VL and stored at − 20 °C. 86 humans at titers of anti-Leishmania antibodies ≥ 1:800 and 90 dog samples at titers of anti-Leishmania antibodies ≥ 1:80 determined by DAT. Moreover, 20 sera were collected from malaria, tuberculosis, cutaneous leishmaniasis, toxoplasmosis and hydatidosis patients and 16 canine sera positive for toxocariasis, toxoplasmosis, cutaneous leishmaniasis and babesiosis for determining of cross-reactivity.
Preparation of mixed recombinant rK39 & rK26 antigens for ELISA
The inserted L. infantum k39 and k26 genes in pET 32a ( +) vectors were used for protein expression in BL21(DE3). The rK39 and rK26 proteins were purified from the soluble fraction by NI-IDA resin affinity chromatography. Western blotting revealed a single band of 58 kDa for rK39 and 50 kDa for rK26. Protein concentration has been estimated 47.8 µgr/ml and 19.8 µgr/ml for rK39 and rK26, respectively after purification and dialysis. All the protein expression and purification procedures were based on the protocols described by Hosseini et al.13, 22.
Preparation of promastigote crude antigen for ELISA
The L. infantum strain (MCAN/IR/14/M14 with GenBank Accession number KT201383) used in this study was isolated from an infected dog with clinical signs from one of the endemic areas for VL in Iran. Spleen biopsy samples were aseptically cultured in RPMI-1640 (Sigma-Aldrich) and 10% inactivated fetal bovine serum (FBS) at 25 ± 1 °C. The late-logarithmic phase of L. infantum promastigotes in the first passage (MCAN/IR/14/M14 with GenBank Accession number KT201383) were sonicated after washing in cold PBS for 3 times. The supernatant containing crude Ag was stored at − 20 °C after centrifugation at 4500 rpm for 20 min at 4 °C. Protein concentration was estimated by the Lowry’s method36.
ELISA assay
rK39 & rK26 antigens from an Iranian strain of L. infantum produced and purified as previously described were aliquoted and stored at − 70 °C until further use13, 22. The concentration of mixed rK39 & rK26 and CA was optimized by a checkerboard titration in standard ELISA methods37.
ELISA plates (Nunc™ MaxiSorp™) were coated overnight with mixed rK39 & rK26 Ag (1 + 1 µg/ml) and crude antigen (6 µg/ml) at 4 °C in carbonate/bicarbonate buffer at pH = 9.6. Then, the protocol was done according to the method Hosseini et al. explained.
The optical density (OD) was entered into MedCalc (Version 17.7.2). The ROC- curve was created for mixed rK39 & rK26 and CA in this software. The best cut-off points were selected to give the maximum sensitivity and specificity.
Direct Agglutination Test (DAT)
Serial dilutions of sera (1:800 to 1:102,400 for human sera in physiological saline (NaCl 0.9%) and 1:80 to 1:20,480 for dog sera in physiological saline (NaCl 0.9%) to which 0.78% and 1.56% β – Mercaptoethanol for human and dog sera respectively, along with negative and positive control sera were added to each well of a V-shaped plate (NUNC, Germany). 50 µl of DAT antigen were added to each well and left overnight at room temperature. The agglutination was checked visually17, 38.
The cut-off point for human and dog sera were considered according to titers described by Hosseini farash et al. (1:800 for human and 1:80 for dog)13.
Statistical analysis
The sensitivity and specificity, positive predictive and negative predictive values were calculated in comparison with DAT. To show the degree of agreement between expected result (mixed rK39 and rK26 Ag ELISA,) and actual result (DAT results), kappa (k) values (95% confidence intervals) were determined based on Landis and Koch criteria scales. The k is less than 0, no agreement, if 0–0.2, slight agreement, if 0.2–0.4, weak agreement, if 0.4–0.6, moderate agreement, if 0.6–0.8, substantial agreement and if 0.8–1.0, almost perfect agreement39. The correlation between mixed rK39 and rK26 Ag ELISA and CA-ELISA results with DAT was evaluated using McNamara's test and analyzed by MedCalc software (Version 17.7.2). Youden's index was calculated to determine the diagnostic efficacy (accuracy), for mixed rK39 and rK26 Ag ELISA and CA-ELISA in comparison with the DAT. The datasets analyzed during the current study are available in the ImmPort Galaxy website and the history is currently accessible by visiting the following URL: https://galaxy.immport.org/u/hoseinifr/h/unnamed-history.
References
Valero, N. N. H. & Uriarte, M. Environmental and socioeconomic risk factors associated with visceral and cutaneous leishmaniasis: A systematic review. Parasitol. Res. 119, 365–384 (2020).
Hajjaran, H. et al. Molecular identification and polymorphism determination of cutaneous and visceral leishmaniasis agents isolated from human and animal hosts in Iran. BioMed. Res. Int. 2013, 789326 (2013).
Mohebali, M. Visceral leishmaniasis in Iran: Review of the epidemiological and clinical features. Iran. J. Parasitol. 8, 348–358 (2013).
Dantas-Torres, F. The role of dogs as reservoirs of Leishmania parasites, with emphasis on Leishmania (Leishmania) infantum and Leishmania (Viannia) Braziliensis. Vet. Parasitol. 149, 139–146 (2007).
Singh, O. P. et al. Xenodiagnosis to evaluate the infectiousness of humans to sandflies in an area endemic for visceral leishmaniasis in Bihar, India: A transmission-dynamics study. Lancet Microbe 2, e23–e31 (2021).
Heidari, S. et al. Identification of immunodominant proteins of Leishmania infantum by immunoproteomics to evaluate a recombinant multi-epitope designed antigen for serodiagnosis of human visceral leishmaniasis. Exp. Parasitol. 222, 108065 (2021).
Karimi Kakh, M., Golchin, M., Kazemi Arababadi, M. & Daneshvar, H. Application of the Leishmania infantum 21-kDa recombinant protein for the development of an immunochromatographic test. Parasite Immunol. 42, e12770 (2020).
Mohebali, M. et al. The diagnostic accuracy of direct agglutination test for serodiagnosis of human visceral leishmaniasis: A systematic review with meta-analysis. BMC Infect. Dis. 20, 946 (2020).
Sundar, S. & Rai, M. Laboratory diagnosis of visceral leishmaniasis. Clin. Diagn. Lab. Immunol. 9, 951–958 (2002).
Elmahallawy, E. K. et al. Diagnosis of leishmaniasis. J. Infect. Dev. Ctries. 8, 961–972 (2014).
Pereira, I. E. et al. Performance of recombinant proteins in diagnosis and differentiation of canine visceral leishmaniasis infected and vaccinated dogs. Eur. J. Microbiol. Immunol. 10, 165–171 (2020).
Chauhan, I. S. et al. Recombinant Leishmania Rab6 (rLdRab6) is recognized by sera from visceral leishmaniasis patients. Exp. Parasitol. 170, 135–147 (2016).
Hosseini Farash, B. R. et al. The rK39 antigen from an Iranian strain of Leishmania infantum: Detection of anti-Leishmania antibodies in humans and dogs. Iran. J. Parasitol. 15, 48–56 (2020).
Hajjaran, H. et al. The geographical distribution of human cutaneous and visceral Leishmania species identified by molecular methods in Iran: A systematic review with meta-analysis. Front. Public Health 9, 835 (2021).
Abass, E. et al. Heterogeneity of Leishmania donovani parasites complicates diagnosis of visceral leishmaniasis: Comparison of different serological tests in three endemic regions. PLoS ONE 10, e0116408 (2015).
Mohapatra, T. M., Singh, D. P., Sen, M. R., Bharti, K. & Sundar, S. Compararative evaluation of rK9, rK26 and rK39 antigens in the serodiagnosis of Indian visceral leishmaniasis. J. Infect. Dev. Ctries. 4, 114–117 (2010).
Farajnia, S. et al. Development and evaluation of Leishmania infantum rK26 ELISA for serodiagnosis of visceral leishmaniasis in Iran. Parasitology 135, 1035–1041 (2008).
Silva, L. A. et al. Antileishmania immunological tests for asymptomatic subjects living in a visceral leishmaniasis-endemic area in Brazil. Am. J. Trop. Med. Hyg. 84, 261–266 (2011).
Moshfe, A. et al. Seroepidemiological study on canine visceral leishmaniasis in Meshkin-Shahr District, Ardabil Province, Northwest of Iran during 2006–2007. Iran. J. Parasitol. 3, 1–10 (2008).
Srivastava, P., Dayama, A., Mehrotra, S. & Sundar, S. Diagnosis of visceral leishmaniasis. Trans. R. Soc. Trop. Med. Hyg. 105, 1–6 (2011).
Rosati, S. et al. Prokaryotic expression and antigenic characterization of three recombinant Leishmania antigens for serological diagnosis of canine leishmaniasis. Clin. Diagn. Lab. Immunol. 10, 1153–1156 (2003).
Hosseini Farash, B. R. et al. Cloning of K26 hydrophilic antigen from Iranian strain of Leishmania infantum. Iran. J. Public Health 46, 1359–1365 (2017).
Soto, M., Requena, J. M., Quijada, L. & Alonso, C. Multicomponent chimeric antigen for serodiagnosis of canine visceral leishmaniasis. J. Clin. Microbiol. 36, 58–63 (1998).
Gontijo, C. M. F. & Melo, M. N. Leishmaniose visceral no Brasil: quadro atual, desafios e perspectivas. Rev. Bras. Epidemiol. 7, 338–349 (2004).
Porrozzi, R. et al. Comparative evaluation of enzyme-linked immunosorbent assays based on crude and recombinant leishmanial antigens for serodiagnosis of symptomatic and asymptomatic Leishmania infantum visceral infections in dogs. Clin. Vaccine Immunol. 14, 544–548 (2007).
Mettler, M., Grimm, F., Capelli, G., Camp, H. & Deplazes, P. Evaluation of enzyme-linked immunosorbent assays, an immunofluorescent-antibody test, and two rapid tests (immunochromatographic-dipstick and gel tests) for serological diagnosis of symptomatic and asymptomatic Leishmania infections in dogs. J. Clin. Microbiol. 43, 5515–5519 (2005).
Quinnell, R. J., Carson, C., Reithinger, R., Garcez, L. M. & Courtenay, O. Evaluation of rK39 rapid diagnostic tests for canine visceral leishmaniasis: Longitudinal study and meta-analysis. PLoS Negl. Trop. Dis. 7, e1992 (2013).
Rosário, E. Y. et al. Evaluation of enzyme-linked immunosorbent assay using crude Leishmania and recombinant antigens as a diagnostic marker for canine visceral leishmaniasis. Mem. Inst. Oswaldo Cruz 100, 197–203 (2005).
Elmahallawy, E. K., Alkhaldi, A. A. M. & Saleh, A. A. Host immune response against leishmaniasis and parasite persistence strategies: A review and assessment of recent research. Biomed. Pharmacother. 139, 111671 (2021).
Edrissian, G. H. et al. Seroepidemiological studies of visceral leishmaniasis and search for animal reservoirs in Fars province, southern Iran. Iran. J Med Sci 18, 99–105 (1993).
Badaró, R., Reed, S. G. & Carvalho, E. M. Immunofluorescent antibody test in American visceral leishmaniasis: Sensitivity and specificity of different morphological forms of two Leishmania species. Am. J. Trop. Med. Hyg. 32, 480–484 (1983).
Boarino, A. et al. Development of recombinant chimeric antigen expressing immunodominant B epitopes of Leishmania infantum for serodiagnosis of visceral leishmaniasis. Clin. Diagn. Lab. Immunol. 12, 647–653 (2005).
Farahmand, M. & Nahrevanian, H. Application of recombinant proteins for serodiagnosis of visceral leishmaniasis in humans and dogs. Iran. Biomed. J. 20, 128–134 (2016).
Mohebali, M., Taran, M. & Zarei, Z. Rapid detection of Leishmania infantum infection in dogs: Comparative study using an immunochromatographic dipstick rk39 test and direct agglutination. Vet. Parasitol. 121, 239–245 (2004).
Mohebali, M. et al. Application of direct agglutination test (DAT) for the diagnosis and seroepide-miological studies of visceral leishmaniasis in Iran. Iran. J. Parasitol. 1, 15–25 (2006).
Lowry, O. H., Rosebrough, N. J., Farr, A. L. & Randall, R. J. Protein measurement with the folin phenol reagent. J. Biol. Chem. 193, 265–275 (1951).
Malla, N. et al. Antigenaemia and antibody response to Leishmania donovani stage-specific antigens and rk39 antigen in human immunodeficiency virus-infected patients. Br. J. Biomed. Sci. 60, 210–216 (2003).
Mohebali, M. et al. Epidemiological aspects of canine visceral leishmaniosis in the Islamic Republic of Iran. Vet. Parasitol. 129, 243–251 (2005).
Landis, J. R. & Koch, G. G. The measurement of observer agreement for categorical data. Biometrics 33, 159–174 (1977).
Acknowledgements
We thank our colleagues from leishmaniasis lab of the School of Public Health, Tehran University of Medical Sciences, the Genetic Lab. of Mashhad University of Medical Sciences and Biotechnology Lab. of Shahid Beheshti University of Medical Sciences who provided insight and expertise that greatly assisted the research. We also thank Alireza Borjian Boroujeni to help us in preparation of some VL negative control sera.
Funding
This study received financial support from Tehran University of Medical Sciences (Project Nos: 92-03-162-24558 and 97-02-160-39164).
Author information
Authors and Affiliations
Contributions
B.R.H.F. performed the experiments and analysed the data. M.M. and B.K. designed the work and supervised the findings of this work. A.F. and H.H. helped B.R.H.F. in performing some processes of this study. B.A. and R.R. contributed to sample preparation. P.M. and B.R.H.F. wrote the paper with input from all authors. E.M. and A.K. and G.S.S. assisted B.R.H.F. in doing experiments. All authors discussed the results and commented on the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Hosseini Farash, B.R., Mohebali, M., Kazemi, B. et al. Validation of a mixture of rK26 and rK39 antigens from Iranian strain of Leishmania infantum to detect anti-Leishmania antibodies in human and reservoir hosts. Sci Rep 12, 10426 (2022). https://doi.org/10.1038/s41598-022-14490-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-022-14490-6
- Springer Nature Limited