Abstract
Purpose
Nowadays, due to the lack of an effective vaccine to prevent the toxoplasmosis, chemotherapy with the combination of pyrimethamine and sulfadiazine is considered as the “gold standard” treatment for toxoplasmosis. Recent reports have exhibited that these synthesized chemical drugs are associated with some serious side effects. The present study aims to evaluate the prophylactic effects of copper nanoparticles (CuNPs) green synthesized by Capparis spinosa fruit methanolic extract alone and combined with atovaquone against chronic toxoplasmosis induced by the Tehran strain of Toxoplasma gondii in mice
Methods
Mice were then orally administrated with CuNPs at the doses of 2 and 4 mg/kg/day and in combined with atovaquone 50 mg/kg for 14 days. Male BALB/c mice were divided into two seven groups include C1 (non-treated non-infected); C2 (treated with normal saline); C3 (Infected mice treated with atovaquone 100 mg/kg/day); Ex1 (treated with CuNPs 2 mg/kg/day); Ex2 (treated with CuNPs 4 mg/kg/day); Ex3 (treated with CuNPs 2 mg/kg/day + atovaquone 50 mg/kg/day); Ex3 (treated with CuNPs 4 mg/kg/day + atovaquone 50 mg/kg/day). On the 15th day, the mice were infected with the intraperitoneal inoculation of 20–25 tissue cysts from the Tehran strain of T. gondii. The mean numbers of brain tissue cysts and the mRNA levels of IL-12, IFN-γ, and inducible nitric oxide synthase (iNOS) in mice of each tested group were measured.
Results
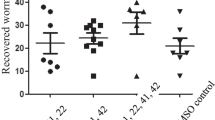
CuNPs were green synthesized by C. spinosa methanolic extract. Scanning electron microscopy showed that the particle size of CuNPs was 17 and 41 nm with maximum peak at the wavelength of 414 nm. The mean number of T. gondii tissue cysts in mice of tested groups of Ex1, Ex2, Ex3, and Ex4, significantly decreased as a dose-dependent response compared with control group. Moreover, in similar to the control group C3, no T. gondii tissue cysts was observed in mice of experimental group Ex3 and Ex4. The mRNA levels of IFN-γ, IL-12, and iNO was measured in mice of all tested groups. The mRNA levels of IFN-γ, IL-12, and iNO was increased in all mice of experimental groups in comparison with the control group C2; however, a significant enhancement was detected in mRNA level of IFN-γ, IL-12, and iNO in the tested groups of Ex3 and Ex4 when compared with control group C3.
Conclusion
The obtained results revealed the high potency of CuNPs alone and combined with atovaquone to prevent toxoplasmosis in mice. Although, the prophylactic effects of CuNPs and other properties, such as improved cellular immunity and low toxicity, are positive topics; however, more studies are required to approve these findings especially in clinical settings.





Similar content being viewed by others
Availability of Data and Materials
All data generated or analyzed during this study are included in this published article.
Change history
12 October 2021
A Correction to this paper has been published: https://doi.org/10.1007/s11686-021-00451-y
References
Mose JM, Kagira JM, Kamau DM, Maina N, Ngotho M, Karanja SM (2020) A review on the present advances on studies of toxoplasmosis in Eastern Africa. Biomed Res Int. https://doi.org/10.1155/2020/7135268
Khryanin A, Reshetniko OV, Kuvshinova IN (2015) Toxoplasmosis: epidemiology, diagnosis, treatment. Antibiot Chemother 60:16–21
Shaapan RM (2016) The common zoonotic protozoal diseases causing abortion. J Parasit Dis 40:1116–1129. https://doi.org/10.1007/s12639-015-0661-5
Elfadaly HA, Hassanain MA, Shaapan RM, Barakat AM, Toaleb NI (2012) Serological and hormonal assays of murine materno-fetal Toxoplasma gondii infection with emphasis on virulent strains. World J Med Sci 7:248–254. https://doi.org/10.5829/idosi.wjms.2012.7.4.6559
Saadatnia G, Golkar M (2012) A review on human toxoplasmosis. Scand J Infect Dis 44(11):805–814. https://doi.org/10.3109/00365548.2012.693197
Artimani T, Shabanian S, Heidari-Soureshjani S, Asadi-Samani M, Luther T (2017) A review of Iranian medicinal plants with teratogenic and abortion-inducing side effects. Int J Pharm Sci Res 8:2372–2377. https://doi.org/10.13040/IJPSR.0975-8232.8(6).2372-77
Montazeri M, Mehrzadi S, Sharif M, Sarvi S, Tanzifi A, Aghaya SA, Daryani A (2018) Drug Resistance in Toxoplasma gondii. Front Microbiol 9:2587. https://doi.org/10.3389/fmicb.2018.02587
Azami SJ, Amani A, Keshavarz H, Najafi-Taher R, Mohebali M, Faramarzi MA, Mahmoudi M, Shojaee S (2018) Nanoemulsion of atovaquone as a promising approach for treatment of acute and chronic toxoplasmosis. Eur J Pharm Sci 117:138–146. https://doi.org/10.1016/j.ejps.2018.02.018
Toaleb NI, Shaapan RM, Hassan SE, El Moghazy FM (2013) High diagnostic efficiency of affinity isolated fraction in camel and cattle toxoplasmosis. World Med Sci J 8:61–66. https://doi.org/10.5829/idosi.wjms.2013.8.1.72161
Rezaei F, Sarvi S, Sharif M, Hejazi SH, Pagheh AS, Aghayan SA, Daryani A (2019) A systematic review of Toxoplasma gondii antigens to find the best vaccine candidates for immunization. Microb Pathog 126:172–184. https://doi.org/10.1016/j.micpath.2018.11.003
Rai M, Ingle AP, Gaikwad S, Gupta I, Gade A, Silvério da Silva S (2016) Nanotechnology based anti-infectives to fight microbial intrusions. J Appl Microbiol 120:527–542. https://doi.org/10.1111/jam.13010
Pelgrift RY, Friedman AJ (2013) Nanotechnology as a therapeutic tool to combat microbial resistance. Adv Drug Deliv Rev 65:1803–1815. https://doi.org/10.1016/j.addr.2013.07.011
Jahangirian H, Lemraski G, Webste TJ, Rafiee-Moghaddam R, Abdollahi Y (2017) A review of drug delivery systems based on nano-technology and green chemistry: green nanomedicine. Int J Nanomed 12:2957. https://doi.org/10.2147/IJN.S127683
Ingl AP, Duran N, Rai M (2014) Bioactivity, mechanism of action, and cytotoxicity of copper-based nanoparticles: a review. Appl Microbiol Biotechnol 98:1001–1009. https://doi.org/10.1007/s00253-013-5422-8
Panda S, Swaminathan S, Hyder KA, Christophel EM, Pendse RN, Sreenivas AN et al (2017) Drug resistance in malaria, tuberculosis, and HIV in South East Asia: biology, programme, and policy considerations. BMJ 358:j3545. https://doi.org/10.1136/bmj.j3545
van Griensven J, Balasegaram M, Meheus F, Alvar J, Lynen L, Boelaert M (2010) Combination therapy for visceral leishmaniasis. Lancet Infect Dis 10:184–194. https://doi.org/10.1016/S1473-3099(10)70011-6
Khatami M, Ebrahimi K, Galehdar N, Moradi MN, Moayyedkazemi A (2020) Green synthesis and characterization of copper nanoparticles and their effects on liver function and hematological parameters in mice. Turkish J Pharm Sci 17(4):412. https://doi.org/10.4274/tjps.galenos.2019.28000
Elfadaly HA, Hassanain NA, Hassanain MA, Barakat AM, Shaapan RM (2018) Evaluation of primitive ground water supplies as a risk factor for the development of major waterborne zoonosis in Egyptian children living in rural areas. J Infect Public Health 11(2):203–208. https://doi.org/10.1016/j.jiph.2017.07.025
Elfadaly HA, Hassanan N, Shaapan RM, Hassanain MA, Barakat AM, Abdelrahman KA (2017) Molecular detection and genotyping of Toxoplasma gondii from Egyptian isolates. Asian J Epidemiol 10:37–44. https://doi.org/10.1016/j.jiph.2017.07.025
Mahmoudvand H, Ziaali N, Ghazvini H, Shojaee S, Keshavarz H, Esmaeilpour K, Sheibani V (2016) Toxoplasma gondii infection promotes neuroinflammation through cytokine networks and induced hyperalgesia in BALB/c mice. Inflammation 39(1):405–412. https://doi.org/10.1007/s10753-015-0262-6
Shaapan RM, Abo-ElMaaty AM, El-Razik KAA, El-Hafez SMA (2012) PCR and serological assays for detection of Toxoplasma gondii infection in sport horses in Cairo. Egypt Asian J Animal Vet Adv 7(2):158–165. https://doi.org/10.3923/ajava.2012.158.165
Ha S, Hamamura MJ, Nalcioglu O, Muftuler LT (2010) A PIN diode controlled dual-tuned MRI RF coil and phased array for multi nuclear imaging. Phys Med Biol 55:2589–2600. https://doi.org/10.1088/0031-9155/55/9/011
Rodriguez JB, Szajnman SH (2012) New antibacterials for the treatment of toxoplasmosis: a patent review. Expert Opin Therap Patents 22(3):311–333. https://doi.org/10.1517/13543776.2012.668886
Ingle AP, Duran N, Rai M (2014) Bioactivity, mechanism of action, and cytotoxicity of copper-based nanoparticles: a review. Appl Microbiol Biotechnol 98(3):1001–1009. https://doi.org/10.1007/s00253-013-5422-8
Thiruvengadam M, Chung IM, Gomathi T, Ansari MA, Khanna VG, Babu V, Rajakumar G (2019) Synthesis, characterization and pharmacological potential of green synthesized copper nanoparticles. Bioprocess Biosyst Eng 42(11):1769–1777. https://doi.org/10.1007/s00449-019-02173-y
Rafique M, Shaikh AJ, Rasheed R, Tahir MB, Bakhat HF, Rafique MS, Rabbani F (2017) A review on synthesis, characterization and applications of copper nanoparticles using green method. NANO 12(04):1750043. https://doi.org/10.1142/S1793292017500436
Al-Hakkani MF (2020) Biogenic copper nanoparticles and their applications: a review. SN Appl Sci 2:505. https://doi.org/10.1007/s42452-020-2279-1
Mahmoodi S, Elmi A, Nezhadi SH (2018) Copper nanoparticles as antibacterial agents. J Mol Pharm. Org Process Res 6:1–7. https://doi.org/10.4172/2329-9053.1000140
Kanhed P, Birla S, Gaikwad S, Gade A, Seabra AB, Rubilar O, Duran N, Rai M (2014) In vitro antifungal efficacy of copper nanoparticles against selected crop pathogenic fungi. Mater Lett 115:13–17. https://doi.org/10.1016/j.matlet.2013.10.011
Saad AHA, Soliman MI, Azzam AM (2015) Antiparasitic activity of silver and copper oxide nanoparticles against Entamoeba histolytica and Cryptosporidium parvum cysts. J Egypt Soc Parasitol 45:593–602. https://doi.org/10.12816/0017920
Malekifard F, Tavassoli M, Vaziri K (2020) In vitro assessment antiparasitic effect of selenium and copper nanoparticles on Giardia duodenalis cyst. Iran J Parasitol 15:411–417. https://doi.org/10.18502/ijpa.v15i3.4206
Albalawi AE, Abdel-Shafy S, Khudair Khalaf A, Alanazi AD, Baharvand P, Ebrahimi K, Mahmoudvand H (2021) Therapeutic potential of green synthesized copper nanoparticles alone or combined with meglumine antimoniate (glucantime®) in cutaneous leishmaniasis. Nanomaterials 11(4):891. https://doi.org/10.3390/nano11040891
Mahmoodi S, Elmi A, Nezhadi SH (2018) Copper nanoparticles as antibacterial agents. J Mol Pharm Org Process Res 6:1–7. https://doi.org/10.4172/2329-9053.1000140
Chatterjee AK, Chakraborty R, Basu T (2014) Mechanism of antibacterial activity of copper nanoparticles. Nanotechnology 25:135101. https://doi.org/10.1088/0957-4484/25/13/135101
Hunter CA, Subauste CS, Remington JS (1994) The role of cytokines in toxoplasmosis. Cytokines Treat Infect Dis. https://doi.org/10.1007/BF01878489
Halonen SK, Chiu FC, Weiss LM (1998) Effect of cytokines on growth of Toxoplasma gondii in murine astrocytes. Infect Immun 66(10):4989–4993. https://doi.org/10.1128/iai.66.10.4989-4993.1998
Douglass ME, Goudie MJ, Pant J, Singha P, Hopkins S, Devine R, Schmiedt CW, Handa H (2019) Catalyzed nitric oxide release via Cu nanoparticles leads to an increase in antimicrobial effects and hemocompatibility for short-term extracorporeal circulation. ACS Appl Bio Mater 2(6):2539–2548. https://doi.org/10.1021/2Facsabm.9b00237
Funding
This research received no external funding.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of Interest
The authors declare that they have no conflict of interests.
Ethics Approval
All required ethical licenses were obtained from the Ethical committee of the Lorestan University of Medical Science (LUMS.REC.1395.178).
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
The original online version of this article was revised: The affiliations were incorrect.
Rights and permissions
About this article
Cite this article
Albalawi, A.E., Alanazi, A.D., Alyousif, M.S. et al. The High Potency of Green Synthesized Copper Nanoparticles to Prevent the Toxoplasma gondii Infection in Mice. Acta Parasit. 66, 1472–1479 (2021). https://doi.org/10.1007/s11686-021-00421-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11686-021-00421-4