Abstract
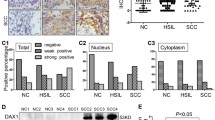
Cervical carcinoma is associated with high propensity for local invasion and lymph node metastasis. However, the molecular alterations that drive progression and metastasis of cervical cancer remain unclear. Cellular senescence has been proposed as the mechanism that protects an organism against cancer progression and metastasis. In addition, Twist, a basic helix-loop-helix transcription factor, has been suggested as an oncogene because it is overexpressed in many types of human cancer. This gene also exhibits a positive function in regulating invasion and metastasis. In this study, Twist was strongly and positively expressed in normal tissue, squamous cell carcinoma (SCC) IA–IIA, and SCC IIB–IIIB (4.3%, 44%, and 88.9%, respectively). The strong positive expressions of the senescence marker CBX3 were 39.1%, 32%, and 15.6%, respectively. The strong positive expressions of Twist in the SCC groups with or without lymph node metastasis were 80.8% and 50%. For CBX3, such expressions were 7.7% and 29.5%, respectively. Results also showed that the expression of Twist was inversely correlated with that of CBX3. Moreover, the knockdown of Twist with target siRNA in SiHa triggered the induction of the chromatin marker of the cellular senescence CBX3 and senescence-associated β-galactosidase activity. Our results suggested that the expression of Twist increased during the progression and metastasis of cervical cancer. Furthermore, Twist-induced senescence bypass is important in this process.
Similar content being viewed by others
References
zur Hausen H. Papillomaviruses and cancer: from basic studies to clinical application. Nat Rev Cancer 2002; 2(5): 342–350
Ozalp S, Yalcin OT, Oner U, Tanir HM, Acikalin M, Sarac I. Microvessel density as a prognostic factor in preinvasive and invasive cervical lesions. Eur J Gynaecol Oncol 2003; 24(5): 425–428
Rodier F, Campisi J. Four faces of cellular senescence. J Cell Biol 2011; 192(4): 547–556
Bartkova J, Rezaei N, Liontos M, Karakaidos P, Kletsas D, Issaeva N, Vassiliou LV, Kolettas E, Niforou K, Zoumpourlis VC, Takaoka M, Nakagawa H, Tort F, Fugger K, Johansson F, Sehested M, Andersen CL, Dyrskjot L, Ørntoft T, Lukas J, Kittas C, Helleday T, Halazonetis TD, Bartek J, Gorgoulis VG. Oncogene-induced senescence is part of the tumorigenesis barrier imposed by DNA damage checkpoints. Nature 2006; 444(7119): 633–637
Huang Y, Myers MP, Xu RM. Crystal structure of the HP1-EMSY complex reveals an unusual mode of HP1 binding. Structure 2006; 14(4): 703–712
Tiwari N, Gheldof A, Tatari M, Christofori G. EMT as the ultimate survival mechanism of cancer cells. Semin Cancer Biol 2012; 22(3): 194–207
Prieur A, Peeper DS. Cellular senescence in vivo: a barrier to tumorigenesis. Curr Opin Cell Biol 2008; 20(2): 150–155
Smit MA, Peeper DS. Epithelial-mesenchymal transition and senescence: two cancer-related processes are crossing paths. Aging (Albany NY) 2010; 2(10): 735–741
Qin Q, Xu Y, He T, Qin C, Xu J. Normal and disease-related biological functions of Twist1 and underlying molecular mechanisms. Cell Res 2012; 22(1): 90–106
Ansieau S, Bastid J, Doreau A, Morel AP, Bouchet BP, Thomas C, Fauvet F, Puisieux I, Doglioni C, Piccinin S, Maestro R, Voeltzel T, Selmi A, Valsesia-Wittmann S, Caron de Fromentel C, Puisieux A. Induction of EMT by twist proteins as a collateral effect of tumorpromoting inactivation of premature senescence. Cancer Cell 2008; 14(1): 79–89
Kwok WK, Ling MT, Yuen HF, Wong YC, Wang X. Role of p14ARF in TWIST-mediated senescence in prostate epithelial cells. Carcinogenesis 2007; 28(12): 2467–2475
Li Y, Wang W, Wang W, Yang R, Wang T, Su T, Weng D, Tao T, Li W, Ma D, Wang S. Correlation of TWIST2 up-regulation and epithelial-mesenchymal transition during tumorigenesis and progression of cervical carcinoma. Gynecol Oncol 2012; 124(1): 112–118
Parkin DM, Bray F, Ferlay J, Pisani P. Estimating the world cancer burden: Globocan 2000. Int J Cancer 2001; 94(2): 153–1
Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin 2011; 61(2): 69–90
Tiwari N, Gheldof A, Tatari M, Christofori G. EMT as the ultimate survival mechanism of cancer cells. Semin Cancer Biol 2012; 22(3): 194–207
Chandeck C, Mooi WJ. Oncogene-induced cellular senescence. Adv Anat Pathol 2010; 17(1): 42–48
Foroni C, Broggini M, Generali D, Damia G. Epithelial-mesenchymal transition and breast cancer: role, molecular mechanisms and clinical impact. Cancer Treat Rev 2012; 38(6): 689–697
Cheng GZ, Chan J, Wang Q, Zhang W, Sun CD, Wang LH. Twist transcriptionally up-regulates AKT2 in breast cancer cells leading to increased migration, invasion, and resistance to paclitaxel. Cancer Res 2007; 67(5): 1979–1987
Lee TK, Poon RT, Yuen AP, Ling MT, Kwok WK, Wang XH, Wong YC, Guan XY, Man K, Chau KL, Fan ST. Twist overexpression correlates with hepatocellular carcinoma metastasis through induction of epithelial-mesenchymal transition. Clin Cancer Res 2006; 12(18): 5369–5376
Lee KW, Kim JH, Han S, Sung CO, Do IG, Ko YH, Um SH, Kim SH. Twist1 is an independent prognostic factor of esophageal squamous cell carcinoma and associated with its epithelialmesenchymal transition. Ann Surg Oncol 2012; 19(1): 326–335
Kajiyama H, Shibata K, Umezu T, Mizuno M, Suzuki S, Yamamoto E, Fujiwara S, Kikkawa F. Expression of Twist enhances risk of poor oncologic outcome in patients with stage Ib to II cervical carcinoma with lymphovascular space involvement. Hum Pathol 2013; 44(2): 181–188
Thiery JP, Acloque H, Huang RY, Nieto MA. Epithelialmesenchymal transitions in development and disease. Cell 2009; 139(5): 871–890
Pedraza-Fariña LG. Mechanisms of oncogenic cooperation in cancer initiation and metastasis. Yale J Biol Med 2006; 79(3–4): 95–103
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Wang, T., Li, Y., Tuerhanjiang, A. et al. Correlation of Twist upregulation and senescence bypass during the progression and metastasis of cervical cancer. Front. Med. 8, 106–112 (2014). https://doi.org/10.1007/s11684-014-0307-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11684-014-0307-5