Abstract
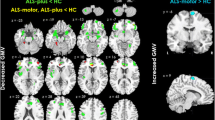
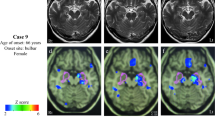
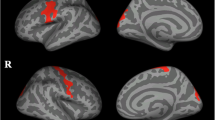
Amyotrophic lateral sclerosis-frontotemporal dementia (ALS-FTD) is rare but exhibits worse prognosis than either ALS or FTD alone. However, cognitive onset ALS-FTD (ALS-FTD-C) confers significantly better patient survival than does motor onset ALS-FTD (ALS-FTD-M), underscoring a meager understanding of pathological group differences. This study aimed to assess disparities in cortical atrophy and perfusion shown by patients with the above disease variants. A total of 38 participants (ALS-FTD-C, 8; ALS-FTD-M, 6; simultaneous-onset ALS-FTD [ALS-FTD-S], 4; healthy controls [HC], 20) qualified for the study and underwent magnetic resonance imaging scan. Three-dimensional T1-weighted structural brain imaging and 3D-pseudocontinuous arterial spin-labeled imaging were routinely collected. Gray matter volume (GMV) and cerebral blood flow (CBF) in ALS-FTD-C and ALS-FTD-M were compared through voxel-based analysis. Correlations between imaging parameters and clinical data were also assessed. Compared with HC, ALS-FTD had significant GMV reduction mainly in bilateral limbic system. GMV reduction in ALS-FTD-C was similar in pattern but less widespread, whereas ALS-FTD-M lacked any significant GMV reduction. In CBF analyses, ALS-FTD displayed hypoperfusion in bilateral motor cortex, frontotemporal lobe, and left basal ganglia. Hypoperfusion involved bilateral temporal lobe, prefrontal cortex, and putamen in ALS-FTD-C but was limited to left parahippocampal gyrus in ALS-FTD-M. Correlations between clinical data and GMV/CBF changes in specific regions were also identified in ALS-FTD. Group-specific patterns of cortical atrophy and perfusion were evident in ALS-FTD-C and ALS-FTD-M. ALS-FTD-C showed pronounced cortical atrophy and hypoperfusion, which were otherwise minimal in ALS-FTD-M. Above findings preliminarily revealed the pathological group differences that may help in classifying patients with ALS-FTD.





Similar content being viewed by others
Data availability
The datasets used and analyzed during the current study are available from the corresponding author on reasonable request.
Code availability
Not applicable.
References
Agosta, F., Valsasina, P., Riva, N., Copetti, M., Messina, M. J., Prelle, A., ..., Filippi, M. (2012). The cortical signature of amyotrophic lateral sclerosis. PLoS One, 7(8), e42816. https://doi.org/10.1371/journal.pone.0042816.
Ahmed, R. M., Devenney, E. M., Strikwerda-Brown, C., Hodges, J. R., Piguet, O., & Kiernan, M. C. (2020). Phenotypic variability in ALS-FTD and effect on survival. Neurology, 94(19), e2005–e2013. https://doi.org/10.1212/WNL.0000000000009398
Ashburner, J., & Friston, K. J. (2000). Voxel-based morphometry--the methods. Neuroimage, 11(6 Pt 1), 805–821. https://doi.org/10.1006/nimg.2000.0582.
Bede, P., Omer, T., Finegan, E., Chipika, R. H., Iyer, P. M., Doherty, M. A., ..., Hardiman, O. (2018). Connectivity-based characterisation of subcortical grey matter pathology in frontotemporal dementia and ALS: a multimodal neuroimaging study. Brain Imaging and Behavior, 12(6), 1696–1707. https://doi.org/10.1007/s11682-018-9837-9.
Buhour, M. S., Doidy, F., Laisney, M., Pitel, A. L., de La Sayette, V., Viader, F., ..., Desgranges, B. (2017a). Pathophysiology of the behavioral variant of frontotemporal lobar degeneration: A study combining MRI and FDG-PET. Brain Imaging and Behavior, 11(1), 240–252. https://doi.org/10.1007/s11682-016-9521-x.
Buhour, M. S., Doidy, F., Mondou, A., Pélerin, A., Carluer, L., Eustache, F., ..., Desgranges, B. (2017b). Voxel-based mapping of grey matter volume and glucose metabolism profiles in amyotrophic lateral sclerosis. EJNMMI Research, 7(1), 21. https://doi.org/10.1186/s13550-017-0267-2.
Burrell, J. R., Kiernan, M. C., Vucic, S., & Hodges, J. R. (2011). Motor Neuron dysfunction in frontotemporal dementia. Brain, 134, 2582–2594. https://doi.org/10.1093/brain/awr195
Burrell, J. R., Halliday, G. M., Kril, J. J., Ittner, L. M., Götz, J., Kiernan, M. C., & Hodges, J. R. (2016). The frontotemporal dementia-motor neuron disease continuum. Lancet, 388(10047), 919–931. https://doi.org/10.1016/s0140-6736(16)00737-6
Cedarbaum, J. M., Stambler, N., Malta, E., Fuller, C., Hilt, D., Thurmond, B., & Nakanishi, A. (1999). The ALSFRS-R: a revised ALS functional rating scale that incorporates assessments of respiratory function. BDNF ALS Study Group (Phase III). Journal of the Neurological Sciences, 169(1–2), 13–21. https://doi.org/10.1016/s0022-510x(99)00210-5
Chen, Y., Kumfor, F., Landin-Romero, R., Irish, M., & Piguet, O. (2019). The Cerebellum in Frontotemporal Dementia: a Meta-Analysis of Neuroimaging Studies. Neuropsychology Review, 29(4), 450–464. https://doi.org/10.1007/s11065-019-09414-7
Consonni, M., Contarino, V. E., Catricala, E., Dalla Bella, E., Pensato, V., Gellera, C., ..., Cappa, S. F. (2018). Cortical markers of cognitive syndromes in amyotrophic lateral sclerosis. Neuroimage Clinical, 19, 675–682. https://doi.org/10.1016/j.nicl.2018.05.020.
Cui, B., Cui, L., Gao, J., Liu, M., Li, X., Liu, C., ..., Fang, J. (2015). Cognitive Impairment in Chinese Patients with Sporadic Amyotrophic Lateral Sclerosis. PLoS One, 10(9), e0137921. https://doi.org/10.1371/journal.pone.0137921.
DeJesus-Hernandez, M., Mackenzie, I. R., Boeve, B. F., Boxer, A. L., Baker, M., Rutherford, N. J., ..., Rademakers, R. (2011). Expanded GGGGCC Hexanucleotide Repeat in Noncoding Region of C9ORF72 Causes Chromosome 9p-Linked FTD and ALS. Neuron, 72(2), 245–256. https://doi.org/10.1016/j.neuron.2011.09.011.
Ferraro, P. M., Jester, C., Olm, C. A., Placek, K., Agosta, F., Elman, L., ..., McMillan, C. T. (2018). Perfusion alterations converge with patterns of pathological spread in transactive response DNA-binding protein 43 proteinopathies. Neurobiology of Aging, 68, 85–92. https://doi.org/10.1016/j.neurobiolaging.2018.04.008.
Folstein, M. F., Folstein, S. E., & McHugh, P. R. (1975). "Mini-mental state". A practical method for grading the cognitive state of patients for the clinician. Journal of Psychiatric Research, 12(3), 189–198. https://doi.org/10.1016/0022-3956(75)90026-6
Guedj, E., Le Ber, I., Lacomblez, L., Dubois, B., Verpillat, P., Didic, M., ..., Habert, M. O. (2007). Brain spect perfusion of frontotemporal dementia associated with motor neuron disease. Neurology, 69(5), 488–490. https://doi.org/10.1212/01.wnl.0000266638.53185.e7
Hu, W. T., Seelaar, H., Josephs, K. A., Knopman, D. S., Boeve, B. F., Sorenson, E. J., ..., Grossman, M. (2009). Survival profiles of patients with frontotemporal dementia and motor neuron disease. Archives of Neurology, 66(11), 1359–1364. https://doi.org/10.1001/archneurol.2009.253
Kansal, K., Mareddy, M., Sloane, K. L., Minc, A. A., Rabins, P. V., McGready, J. B., & Onyike, C. U. (2016). Survival in Frontotemporal Dementia Phenotypes: A Meta-Analysis. Dementia and Geriatric Cognitive Disorders, 41(1–2), 109–122. https://doi.org/10.1159/000443205
Lomen-Hoerth, C., Anderson, T., & Miller, B. (2002). The overlap of amyotrophic lateral sclerosis and frontotemporal dementia. Neurology, 59(7), 1077–1079. https://doi.org/10.1212/wnl.59.7.1077
Machts, J., Loewe, K., Kaufmann, J., Jakubiczka, S., Abdulla, S., Petri, S., ..., Bede, P. (2015). Basal ganglia pathology in ALS is associated with neuropsychological deficits. Neurology, 85(15), 1301–1309. https://doi.org/10.1212/wnl.0000000000002017.
Marin, B., Boumédiene, F., Logroscino, G., Couratier, P., Babron, M. C., Leutenegger, A. L., ..., Beghi, E. (2017). Variation in worldwide incidence of amyotrophic lateral sclerosis: a meta-analysis. International Journal of Epidemiology, 46(1), 57–74. https://doi.org/10.1093/ije/dyw061
Montuschi, A., Iazzolino, B., Calvo, A., Moglia, C., Lopiano, L., Restagno, G., ..., Chiò, A. (2015). Cognitive correlates in amyotrophic lateral sclerosis: a population-based study in Italy. Journal of Neurology, Neurosurgery, and Psychiatry, 86(2), 168–173. https://doi.org/10.1136/jnnp-2013-307223
Müller-Gärtner, H. W., Links, J. M., Prince, J. L., Bryan, R. N., McVeigh, E., Leal, J. P., ..., Frost, J. J. (1992). Measurement of radiotracer concentration in brain gray matter using positron emission tomography: MRI-based correction for partial volume effects. Journal of Cerebral Blood Flow and Metabolism, 12(4), 571–583. https://doi.org/10.1038/jcbfm.1992.81
Murphy, J., Factor-Litvak, P., Goetz, R., Lomen-Hoerth, C., Nagy, P. L., Hupf, J., ..., Mitsumoto, H. (2016). Cognitive-behavioral screening reveals prevalent impairment in a large multicenter ALS cohort. Neurology, 86(9), 813–820. https://doi.org/10.1212/wnl.0000000000002305
Nasreddine, Z. S., Phillips, N. A., Bédirian, V., Charbonneau, S., Whitehead, V., Collin, I., ..., Chertkow, H. (2005). The Montreal Cognitive Assessment, MoCA: a brief screening tool for mild cognitive impairment. Journal of the American Geriatrics Society, 53(4), 695–699. https://doi.org/10.1111/j.1532-5415.2005.53221.x.
Neumann, M., Sampathu, D. M., Kwong, L. K., Truax, A. C., Micsenyi, M. C., Chou, T. T., ..., Lee, V. M. Y. (2006). Ubiquitinated TDP-43 in frontotemporal lobar degeneration and amyotrophic lateral sclerosis. Science, 314(5796), 130–133. https://doi.org/10.1126/science.1134108
Niccoli, T., Partridge, L., & Isaacs, A. M. (2017). Ageing as a risk factor for ALS/FTD. Human Molecular Genetics, 26(R2), R105–r113. https://doi.org/10.1093/hmg/ddx247
Pizzarotti, B., Palesi, F., Vitali, P., Castellazzi, G., Anzalone, N., Alvisi, E., ..., Gandini Wheeler-Kingshott, C. A. M. (2020). Frontal and Cerebellar Atrophy Supports FTSD-ALS Clinical Continuum. Frontiers in Aging Neuroscience, 12, 593526. https://doi.org/10.3389/fnagi.2020.593526
Prell, T., & Grosskreutz, J. (2013). The involvement of the cerebellum in amyotrophic lateral sclerosis. Amyotroph Lateral Scler Frontotemporal Degener, 14(7–8), 507–515. https://doi.org/10.3109/21678421.2013.812661
Rajagopalan, V., & Pioro, E. P. (2014). Distinct patterns of cortical atrophy in ALS patients with or without dementia: an MRI VBM study. Amyotroph Lateral Scler Frontotemporal Degener, 15(3–4), 216–225. https://doi.org/10.3109/21678421.2014.880179
Rajagopalan, V., & Pioro, E. P. (2015). Comparing brain structural MRI and metabolic FDG-PET changes in patients with ALS-FTD: 'the chicken or the egg?' question. Journal of Neurology, Neurosurgery, and Psychiatry, 86(9), 952–958. https://doi.org/10.1136/jnnp-2014-308239
Rajagopalan, V., & Pioro, E. P. (2020). 2-Deoxy-2-[(18) F]fluoro-d-glucose positron emission tomography, cortical thickness and white matter graph network abnormalities in brains of patients with amyotrophic lateral sclerosis and frontotemporal dementia suggest early neuronopathy rather than axonopathy. European Journal of Neurology. https://doi.org/10.1111/ene.14332
Ramasubbu, R., Brown, E. C., Marcil, L. D., Talai, A. S., & Forkert, N. D. (2019). Automatic classification of major depression disorder using arterial spin labeling MRI perfusion measurements. Psychiatry and Clinical Neurosciences, 73(8), 486–493. https://doi.org/10.1111/pcn.12862
Ratti, E., Domoto-Reilly, K., Caso, C., Murphy, A., Brickhouse, M., Hochberg, D., ..., Dickerson, B. C. (2021). Regional prefrontal cortical atrophy predicts specific cognitive-behavioral symptoms in ALS-FTD. Brain Imaging and Behavior, 15(5), 2540–2551. https://doi.org/10.1007/s11682-021-00456-1
Renard, D., Collombier, L., Castelnovo, G., Fourcade, G., Kotzki, P. O., & LaBauge, P. (2011). Brain FDG-PET changes in ALS and ALS-FTD. Acta Neurologica Belgica, 111(4), 306–309.
Renton, A. E., Majounie, E., Waite, A., Simon-Sanchez, J., Rollinson, S., Gibbs, J. R., ..., Consortium, I. (2011). A Hexanucleotide Repeat Expansion in C9ORF72 Is the Cause of Chromosome 9p21-Linked ALS-FTD. Neuron, 72(2), 257–268. https://doi.org/10.1016/j.neuron.2011.09.010
Ringholz, G. M., Appel, S. H., Bradshaw, M., Cooke, N. A., Mosnik, D. M., & Schulz, P. E. (2005). Prevalence and patterns of cognitive impairment in sporadic ALS. Neurology, 65(4), 586–590. https://doi.org/10.1212/01.wnl.0000172911.39167.b6
Shelley, B. P., & Trimble, M. R. (2004). The insular lobe of Reil--its anatamico-functional, behavioural and neuropsychiatric attributes in humans--a review. The World Journal of Biological Psychiatry, 5(4), 176–200. https://doi.org/10.1080/15622970410029933.
Shen, D., Hou, B., Xu, Y., Cui, B., Peng, P., Li, X., ..., Cui, L. (2018). Brain Structural and Perfusion Signature of Amyotrophic Latera Sclerosis With Varying Levels of Cognitive Deficit. Frontiers in Neurology, 9. https://doi.org/10.3389/fneur.2018.00364
Strong, M. J., Abrahams, S., Goldstein, L. H., Woolley, S., McLaughlin, P., Snowden, J., ..., Turner, M. R. (2017). Amyotrophic lateral sclerosis - frontotemporal spectrum disorder (ALS-FTSD): Revised diagnostic criteria. Amyotrophic Lateral Sclerosis and Frontotemporal Degeneration, 18(3–4), 153–174. https://doi.org/10.1080/21678421.2016.1267768
Tan, R. H., Devenney, E., Dobson-Stone, C., Kwok, J. B., Hodges, J. R., Kiernan, M. C., ..., Hornberger, M. (2014). Cerebellar integrity in the amyotrophic lateral sclerosis-frontotemporal dementia continuum. PLoS One, 9(8), e105632. https://doi.org/10.1371/journal.pone.0105632
Tan, J. P., Li, N., Gao, J., Wang, L. N., Zhao, Y. M., Yu, B. C., ..., Zhou, P. Y. (2015). Optimal cutoff scores for dementia and mild cognitive impairment of the Montreal Cognitive Assessment among elderly and oldest-old Chinese population. Journal of Alzheimer's Disease, 43(4), 1403–1412. https://doi.org/10.3233/jad-141278
Verfaillie, S. C., Adriaanse, S. M., Binnewijzend, M. A., Benedictus, M. R., Ossenkoppele, R., Wattjes, M. P., ..., Barkhof, F. (2015). Cerebral perfusion and glucose metabolism in Alzheimer's disease and frontotemporal dementia: two sides of the same coin? European Radiology, 25(10), 3050–3059. https://doi.org/10.1007/s00330-015-3696-1
Wang, H., Li, S., Chen, X., Wang, Y., Li, J., & Wang, Z. (2020). Cerebral Blood Flow Alterations in High Myopia: An Arterial Spin Labeling Study. Neural Plasticity, 2020, 6090262. https://doi.org/10.1155/2020/6090262
Acknowledgements
We thank the ALS-FTD patients and healthy controls for participating in this study.
Funding
This study was supported by the National Natural Science Foundation of China (Grant number: 81801277) and the Strategic Priority Research Program of the Chinese Academy of Sciences (Grant number: XDB39000000).
Author information
Authors and Affiliations
Contributions
YW and DS: study concept and design, data acquisition, statistical analysis, data interpretation, drafting of the manuscript. BH: data acquisition, statistical analysis, critical revision of the manuscript. XS, XY, JG, and ML: data acquisition, study supervision, critical revision of the manuscript. FF: study concept and design, data interpretation, critical revision of the manuscript. LC: study concept and design, data acquisition, data interpretation, study supervision, critical revision of the manuscript.
Corresponding author
Ethics declarations
Ethics approval
This study was approved by the Research Ethics Committee of Peking Union Medical College Hospital (No. JS-2509).
Consent to participate
Written informed consent was obtained in accordance with the Declaration of Helsinki (1991) from each patient or their guardians.
Consent for publication
Not applicable.
Conflicts of interest
All authors declared no conflicts of interest.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
ESM 1
(DOCX 50 kb)
Rights and permissions
About this article
Cite this article
Wang, Y., Shen, D., Hou, B. et al. Brain structural and perfusion changes in amyotrophic lateral sclerosis-frontotemporal dementia patients with cognitive and motor onset: a preliminary study. Brain Imaging and Behavior 16, 2164–2174 (2022). https://doi.org/10.1007/s11682-022-00686-x
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11682-022-00686-x