Abstract
Motor training is a widely used therapy in many pain conditions. The brain’s capacity to undergo functional and structural changes i.e., neuroplasticity is fundamental to training-induced motor improvement and can be assessed by transcranial magnetic stimulation (TMS). The aim was to investigate the impact of pain on training-induced motor performance and neuroplasticity assessed by TMS. The review was carried out in accordance with the PRISMA-guidelines and a Prospero protocol (CRD42020168487). An electronic search in PubMed, Web of Science and Cochrane until December 13, 2019, identified studies focused on training-induced neuroplasticity in the presence of experimentally-induced pain, 'acute pain' or in a chronic pain condition, 'chronic pain'. Included studies were assessed by two authors for methodological quality using the TMS Quality checklist, and for risk of bias using the Newcastle–Ottawa Scale. The literature search identified 231 studies. After removal of 71 duplicates, 160 abstracts were screened, and 24 articles were reviewed in full text. Of these, 17 studies on acute pain (n = 7) or chronic pain (n = 10), including a total of 258 patients with different pain conditions and 248 healthy participants met the inclusion criteria. The most common types of motor training were different finger tasks (n = 6). Motor training was associated with motor cortex functional neuroplasticity and six of seven acute pain studies and five of ten chronic pain studies showed that, compared to controls, pain can impede such trainings-induced neuroplasticity. These findings may have implications for motor learning and performance and with putative impact on rehabilitative procedures such as physiotherapy.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Neuroplasticity can be defined as the brain’s ability to reorganize or undergo functional and structural changes. It was long postulated that neuroplasticity was limited only to the critical period during brain development (Michelini & Stern, 2009). However, during the past decades, it has been widely recognized that neuroplasticity is the normal ongoing state of the human brain throughout the life span (Pascual-Leone et al., 2005). This feature of the brain is considered as one of the foundations for acquisition of new motor skills. In everyday life, we use different motor skills that we have acquired gradually through training and changes in our environment, e.g., walking, driving a car, riding a bicycle or chewing food (Chang, 2014; Lohse et al., 2014).
The neuroplastic changes associated with the training of a skill, i.e., motor training-induced neuroplasticity, are thought to play an important role in the performance of the skill being trained and can be reflected in measures such as accuracy, precision and speed. It has been suggested that training-induced neuroplasticity may be dependent on a number of factors, including the complexity of the skills being trained, training time, motivational conditions, age and the muscle groups activated during skill training to name a few (Duchateau et al., 2006; Hellmann et al., 2011; Wulf et al., 2010).
There are also reports that the presence of pain can have an impact on training-induced neuroplasticity and on the motor performance when executing a motor skill (Bank et al., 2013; Hodges & Tucker, 2011). The effect pain has on motor performance can both be subtle, e.g., redistribution of activity within/between muscles, increased variability, and more salient, e.g., avoidance of the motor behaviour causing or increasing the pain (Akhter et al., 2014; Hodges & Smeets, 2015; Hodges & Tucker, 2011).
Chronic pain conditions, as for example chronic lower back pain, which often result in limitations in motor performance, are common conditions and conventional treatments often involve different types of skill training to reduce disability and pain. Movements engaged during skill training will activate different corticomotor pathways in the brain to achieve appropriate, precise, and effective motor control and facilitate rehabilitation of the motor performance (Gurevich et al., 1994; Kosek et al., 2013). However, conventional rehabilitation and skill training programs may not optimally restore impaired motor performance in patients with chronic pain, compared to pain-free patients, due to a negative effect on training-induced neuroplasticity. Despite the importance of this topic in rehabilitation medicine, the effect of pain on training-induced neuroplasticity and the subsequential impact this has on motor skill acquisition and improvement, is not well understood.
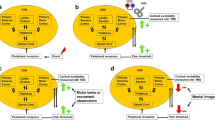
Transcranial magnetic stimulation (TMS), a non-invasive brain imaging technique, can be used to assess training-induced neuroplasticity in corticomotor pathways following motor skill training (Rothwell, 2018). There is some evidence that both acute and chronic pain can affect the plasticity of the motor cortex—as assessed by TMS—following motor skill training, although reported findings are not conclusive. Thus, a reduction in corticomotor excitability has been reported for patients with both chronic and acute pain (Dettmers et al., 2001; Krause et al., 2006). In contrast, both chronic pain (e.g., phantom limb pain) (Dettmers et al., 2001) and acute pain have also been shown to increase motor cortex excitability under certain circumstances (Romaniello et al., 2000).
The aim of this systematic review was to investigate the impact of pain (acute or chronic) on training-induced motor performance and neuroplasticity as assessed by TMS.
Materials and methods
Protocol
This study followed a protocol that was registered in Prospero (CRD42020168487) and was carried out in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) Statement (Moher et al., 2009).
Inclusion and exclusion criteria
Eligibility criteria were formulated using PICO (population, intervention, comparison and outcome) to identify clinical studies published in English and focused on pain and training-induced neuroplasticity assessed with TMS in humans.
-
Populations: Healthy humans with experimentally induced acute pain or humans with a chronic pain condition.
-
Intervention: Short-term motor task training in the presence of pain (acute or chronic).
-
Comparison: Healthy humans with no pain.
-
Primary outcome: Neuroplasticity assessed with TMS targeting motor cortex areas of the corresponding muscles involved in the training task performed in the presence of pain.
-
Secondary outcome: Behavioral and functional outcomes.
Articles were excluded if a) the population had psychiatric or neurological disorders, b) participants did not perform an active form of training, c) participants performed long term training (e.g., sport athletes) and d) the training was combined with other interventions (e.g., paired associative stimulation).
Literature search
An electronic search was carried out in PubMed, Cochrane and Web of Science until December 13, 2019. The search strategy was developed for PubMed with a combination of MeSH terms and free text terms in cooperation with an experienced research librarian (Martina Vall) and then adapted to both Cochrane and Web of Science. The search was designed to identify studies that assessed the possible effect of acute or chronic pain on training-induced neuroplasticity assessed with TMS. Table 1 provides the full search strategy for PubMed. There was no limitation on study design or language in the search. The electronic search was combined with a hand search of the reference lists of the included articles to identify additional studies. Grey literature, letters to the editor and editorials were not included, and authors were not contacted for additional information.
Article selection
Two authors (BHH, NS) independently screened all titles and abstracts for potential eligibility. If at least one author deemed an article to be of potential interest, it was retained for the full text assessment. The full text assessment was carried out independently by the same authors to determine if articles met the inclusion criteria. Any disagreements were resolved by discussion between the investigators and if needed by a third author (MK).
Data extraction
Data extraction was carried out by two authors (NS, MK) and checked by a third author (BHH). The data extracted from the individual studies were: first author, publication year, aim, study population, study setting, study design, methods, outcomes, and study summary. All data supporting the findings of this study are available within the article and its 19. A record of the database searches is available on Prospero (CRD42020168487). If results allowed, primarily with regard to reported TMS outcome measures for neuroplasticity and elapsed time between training and assessment, a meta-analysis was planned using a random effects model and using the I2 statistic for assessing heterogeneity among studies.
Quality assessment
Assessment of risk of bias in the included studies was carried out by two authors (NS, LAA) using the Newcastle–Ottawa Scale (NOS) for case–control studies (Stang, 2010). In addition, the included studies were assessed by two authors (NS, YC) with the TMS Quality checklist (Chipchase et al., 2012). For both of these instruments, any disagreement between the authors were resolved by discussion, and if needed by a third author (BHH). The NOS evaluates the risk of bias by looking at eight items categorized in three different domains: selection, comparability and exposure. NOS uses a star system where the studies with the highest quality are awarded a maximum of one star for each item with exception for the item related to comparability where the assignment of two stars are allowed providing a total score between 0 and 9. The TMS Quality checklist includes eight items on participant characteristics, 20 items on TMS methodology, of which three items specifically concern paired pulse only, and two items on analysis. In total, the checklist includes 26 factors that can be reported and/or controlled for in studies using single pulse TMS, and 29 factors that can be reported and/or controlled for in studies using paired pulse TMS. The checklist items were assessed as: “Yes”, “No”, or “Not applicable”, and the number of these respective answers was reported together with the total percentage of “Yes” answers with the number of attainable criteria as denominator.
Results
Literature search
The electronic literature search in PubMed, Cochrane and Web of Science, identified a total of 231 articles (Fig. 1). After removal of 71 duplicates, 160 unique abstracts were screened, and 24 articles were reviewed in full text. Of the 24 articles, 7 articles were excluded (Bradnam et al., 2016; Daligadu et al., 2013; Massé‐Alarie et al., 2015; Massé-Alarie et al., 2017a; McCambridge et al., 2018; Rittig-Rasmussen et al., 2013; Volz et al., 2012) (Table 2) and 17 articles published between 2007 and 2018 met the inclusion criteria, i.e., reported the impact of pain on training-induced neuroplasticity assessed by TMS (Table 3). The electronic search was complemented with a hand search of the reference lists of included articles.
The majority of the included articles focused on chronic pain conditions (n = 10) (Baarbe et al., 2018; Hoeger Bement et al., 2014; Masse-Alarie et al., 2016, 2017b; Mendonca et al., 2016; Parker et al., 2017; Rittig-Rasmussen et al., 2014a; Schwenkreis et al., 2011; Tsao et al., 2010; Vallence et al., 2013), and the remaining articles focused on acute pain in healthy individuals (n = 7) (Boudreau et al., 2007; Dancey et al., 2019; De Martino, Petrini, et al., 2018; De Martino, Zandalasini, et al., 2018; Ingham et al., 2011; Mavromatis et al., 2017; Rittig-Rasmussen et al., 2014b). The chronic pain conditions included headache (n = 1), pain in the neck region (n = 2), lower back pain (n = 3), painful hand arthritis (n = 1) and fibromyalgia (n = 3).
Acute pain was induced by either: i) applying capsaicin cream (n = 3) intraorally (Boudreau et al., 2007), on the right thumb (Mavromatis et al., 2017), or on the dominant elbow (Dancey et al., 2019); ii) by injecting hypertonic saline (n = 2) into the right first dorsal interosseous muscle (Ingham et al., 2011) or the right side of the neck (Rittig-Rasmussen et al., 2014b); or iii) eccentric exercise to induce delayed onset muscle soreness (n = 2): in wrist extensor muscles (De Martino, Petrini, et al., 2018; De Martino, Zandalasini, et al., 2018).
Regarding the training paradigms, only one study focused on the trigeminally-innervated region and involved training in a tongue-protrusion task (Boudreau et al., 2007). The remaining studies focused mainly on the spinally-innervated upper extremities (hands/arms) and most often involved training in different finger tasks (n = 6). These finger tasks included: typing letter sequences on a keyboard (Baarbe et al., 2018), tracing sinusoidal waves on a touchpad with dominant thumb (Dancey et al., 2019), brisk movements with the index finger in the opposite direction to the twitches evoked by TMS (Ingham et al., 2011), pinching a force transducer (Mavromatis et al., 2017), voluntary finger twitching (Parker et al., 2017) and thumb abduction in response to an auditory cue (Vallence et al., 2013).
Six of the seven acute pain studies (Boudreau et al., 2007; Dancey et al., 2019; De Martino, Petrini, et al., 2018; De Martino, Zandalasini, et al., 2018; Mavromatis et al., 2017; Rittig-Rasmussen et al., 2014b) and five of the ten chronic pain studies (Hoeger Bement et al., 2014; Masse-Alarie et al., 2016; Rittig-Rasmussen et al., 2014a; Schwenkreis et al., 2011; Vallence et al., 2013) showed that acute and chronic pain impede trainings-induced functional neuroplasticity otherwise observed in the primary motor cortex (e.g., increased corticomotor excitability).
In terms of motor performance, only five of the seven studies that induced acute pain evaluated subsequent changes in motor performance (Boudreau et al., 2007; Dancey et al., 2019; Ingham et al., 2011; Mavromatis et al., 2017; Rittig-Rasmussen et al., 2014b). Two of these five studies (Boudreau et al., 2007; Ingham et al., 2011) showed that pain in the region being trained (i.e., tongue and finger, respectively) had a negative effect on the training-induced motor performance gain (i.e., tongue protrusion and finger abduction, respectively). The remaining three studies (Dancey et al., 2019; Mavromatis et al., 2017; Rittig-Rasmussen et al., 2014b) did not demonstrate any differences between pain and control groups.
For the chronic pain studies, only five of the ten studies (De Martino, Petrini, et al., 2018; De Martino, Zandalasini, et al., 2018; Hoeger Bement et al., 2014; Mendonca et al., 2016; Schwenkreis et al., 2011) evaluated motor performance in comparison to pain-free control groups. Four of these studies did not show any conclusive findings whereas one study on chronic headache showed greater improvement in a thumb abduction motor task in the pain-free control group (Vallence et al., 2013).
Risk of bias assessment
The risk of bias as assessed with the use of NOS is presented in Table 4. Lower scores were generally associated with study design and often with lack of inadequate control groups. Therefore, we added a modified score for internal control groups. For the assessment with the TMS checklist the average quality score was 72% (range 54—90%), for reported factors and 65% (range 38 – 83%) for controlled factors (Table 5). All studies scored over 50% for factors that should be reported and only three studies scored less than 50% (Baarbe et al., 2018; Hoeger Bement et al., 2014; Mendonca et al., 2016) for factors that should be controlled. However, none of the included studies were rated as having an overall high risk of bias and all studies were retained for the qualitative synthesis.
Meta-analysis
When all included papers were assessed for the possibility of a quantitative analysis, there was a lack of studies reporting central and dispersion measures for comparable outcomes. Two studies from the same research group (Masse-Alarie et al., 2016, 2017b) in chronic back pain patients reported short intracortical inhibition (SICI) and facilitation (SICF) (before and after intervention). The same studies, together with one study on acute pain in healthy individuals (De Martino, Zandalasini, et al., 2018), presented data on MEP amplitudes and silent periods. Based on this, it was not deemed suitable to carry out a meta-analysis but instead a qualitative synthesis of main findings is presented (Table 6).
Discussion
Pain, training, and neuroplasticity
This systematic review included 7 studies investigating the impact of experimental acute pain in pain-free participants, and 10 studies investigating the effects of chronic pain on corticomotor excitability of the muscle in pain and the associated training-induced motor performance gain. The main findings suggest that both acute and chronic pain may impede training-induced neuroplasticity.
The studies included in the present review were heterogeneous with regard to several aspects of study designs. One aspect to consider is that the selected cases included in the studies represented a wide range of chronic pain conditions and different acute pain protocols, manifested at different regions of the body. The chronic pain conditions included either widespread pain in multiple anatomical locations (i.e., fibromyalgia), regional pain in the cervical, spinal, or lumbar regions, or localized pain in the hand, head and facial regions. The acute pain protocols ranged from application of a topical capsaicin cream or hypertonic saline injection to the target muscle or nearby area, to injections of a nerve growth factor, eccentric exercises to provoke delayed onset muscle soreness, or a combination of the last two. Furthermore, the training tasks differed between studies. Some studies were simpler and involved only one (i.e., one-dimensional) or a few muscles, whereas other studies were more complex with tasks involving coordinated activation of multiple muscles and muscle groups as well as being more cognitively demanding. The range of training tasks included some that can be presumed, at least in part, to utilize already existing motor pattern skills such as typing (Baarbe et al., 2018), whereas other motor tasks can be viewed as more novel motor patterns, e.g., tracing an external target utilizing shoulder muscles (Rittig-Rasmussen et al., 2014a, 2014b). Other studies incorporated more fatiguing or strength-demanding tasks (De Martino, Petrini, et al., 2018; De Martino, Zandalasini, et al., 2018; Hoeger Bement et al., 2014; Mavromatis et al., 2017), thereby putting additional functional demands on muscles, muscle groups and the corticospinal tract.
The large variation in the duration, repetition, intensity and type of training performed, each of which can impact the neuroplastic changes occurring in the brain, is consistent with the time, intensity, repetition and specificity principles of neuroplasticity (Avivi-Arber & Sessle, 2018; Kleim & Jones, 2008). This relates to the question “when is enough enough?” or maybe a ceiling effect for pain conditions. It has been shown that neuroplastic changes are more pronounced in skills training (Pascual-Leone et al., 1995), as opposed to fatiguing and strength training exercises (Remple et al., 2001) and that exercise-induced fatigue may even reduce neuroplasticity (Wang et al., 2020). In the present review, several of the primary studies included training protocols either designed to induce fatigue (De Martino, Petrini, et al., 2018; De Martino, Zandalasini, et al., 2018; Hoeger Bement et al., 2014; Mendonca et al., 2016; Schwenkreis et al., 2011), or protocols that may have induced fatigue due to the length of training sessions or the load applied (Boudreau et al., 2007; Parker et al., 2017; Rittig-Rasmussen et al., 2014a, 2014b; Vallence et al., 2013). In addition to the duration of individual sessions, the number of sessions may also affect the overall effects from training, consistent with the ‘repetition’ principle of neuroplasticity.
Consistent with the ‘timing’ principle of neuroplasticity (Avivi-Arber & Sessle, 2018; Kleim & Jones, 2008), the time between training and TMS measurements should be considered in relation to immediate versus more long-lasting neuroplastic changes. Time is an important factor since different forms and different directions of neuroplastic changes occur at different points of time after motor training (Avivi-Arber & Sessle, 2018; Kleim & Jones, 2008). Whereas most studies performed TMS measurement to assess neuroplastic changes immediately after training (i.e., within one-hour timeframe), several studies also explored training-induced neuroplastic changes more thoroughly by performing multiple TMS measurements within the first 15-min (Ingham et al., 2011; Parker et al., 2017; Vallence et al., 2013) or first hour (Rittig-Rasmussen et al., 2014a, 2014b; Schwenkreis et al., 2011; Vallence et al., 2013) following training. Two studies also investigated possible long term effects after one week of training (Rittig-Rasmussen et al., 2014a, 2014b) and found a long lasting inhibition of corticomotor excitability in healthy subjects who performed neck exercises in the presence of acute neck pain (Rittig-Rasmussen et al., 2014b). Therefore, it is noteworthy in the context of training, that pain can induce fast-onset and long-lasting neuroplastic changes manifested as decreased corticomotor excitability. Clearly, there are several important training-related parameters that need to be taken into consideration before a more comprehensive understanding of the effects of pain on training-induced corticomotor excitability can be obtained.
The wide range of pain conditions discussed above can explain variations across the studies in the expression of pain sensations in terms of intensity and quality of pain, as well as temporal aspects of pain such as timing of peak intensity and duration. Consistent with the ‘specificity’ principle of neuroplasticity (Avivi-Arber & Sessle, 2018; Kleim & Jones, 2008), these variations in pain characteristics may explain differences in neuroplastic changes between selected cases and controls within and across studies.
Additionally, age is a factor that should be considered as aging is associated with different processes, e.g., physiological degradation and neuronal atrophy, resulting in declines in sensorimotor control and performance. There was a large mean age range from 21.1 years (Baarbe et al., 2018) to 72.0 years (Parker et al., 2017) for the studies evaluating the effect of chronic pain on corticomotor excitability, whereas for the studies that induced acute pain in pain-free participants the mean age ranged only from 20.2 years (Dancey et al., 2019) to 26.5 years (Mavromatis et al., 2017). However, no significant effects attributed to age were found for any of the included studies. Another subject related factor worth taking into consideration is that of gender. All studies reported varying ratios of male and female participants. According to the TMS checklist gender is a factor that only should be reported and not controlled, which was the case for all included studies.
As mentioned earlier, most studies incorporated training that targeted painful areas, either through acute pain or by the presence of localised chronic pain conditions. There was however a number of studies that examined the more global effects, from chronic tension type headaches (Vallence et al., 2013), neck pain (Baarbe et al., 2018), back pain (Tsao et al., 2010) or fibromyalgia (Schwenkreis et al., 2011) in hand or arm training or from knee pain in neck training (Rittig-Rasmussen et al., 2014a). The last of these studies investigated neck training in three groups; neck pain, knee pain and controls, and demonstrated significantly reduced corticomotor excitability of neck muscles as assessed by MEP amplitudes in the neck pain, but not in the knee pain or control groups (Rittig-Rasmussen et al., 2014a). This finding is in accordance with a lack of difference in MEP amplitudes between healthy controls and a neck pain group following a typing task (Baarbe et al., 2018), and between healthy controls and a fibromyalgia group following a hand grip task (Schwenkreis et al., 2011). This finding strongly indicates that it may not be pain in general but pain in a relevant region for the motor task that determines the impact on training-induced neuroplasticity. In contrast, reduced motor learning in a thumb abduction task was reported for headache patients compared to healthy controls (Vallence et al., 2013), indicating that the relationship between pain location and trained regions may be dependent not only on the location but also on the type of pain condition.
There are several factors to consider when comparing the chronic pain and acute pain groups of the studies included in the present review. One aspect is that in the chronic pain conditions, and in most of the acute pain conditions, motor skill acquisition training and motor skill acquisition occurred in the presence of pain. In contrast, in two studies of acute pain, motor training occurred in the absence of pain but the motor skill acquisition was evaluated in the presence of pain (De Martino, Petrini, et al., 2018; De Martino, Zandalasini, et al., 2018). In a systematic review based on 43 studies that evaluated motor cortex excitability in chronic pain conditions, Parker et al. found that chronic pain conditions can induce a range of motor cortex neuroplastic changes that vary across studies there was inconsistency for most outcome measures. Among the changes were reduced duration of silent period and SICI together with enhanced SICF. There were also indications that these effects were more pronounced in populations with neuropathic pain (Parker et al., 2016). Most of the included studies were however based on migraine populations, thereby representing a pain condition with complex pathophysiology specifically related to the trigeminal region.
Functional aspects
Another factor to consider when comparing exercise in the presence of chronic pain versus acute pain is the “salience principle” (Kleim & Jones, 2008). The salience principle describes the brain's attention to any input, and in the context of exercise can be seen as increased attention to a task being practiced. Patients with chronic pain may have such a cognitive incentive when exercising, due to perceived or expected healing or analgesic effects. In acute pain, however, exercise probably has no cognitive incentive, but rather may have an “interference” effect, e.g., worsening the acute pain, thereby resulting in non-salient exercise. This is supported by the increased representation in the motor cortex from salient exercise compared to non-salient exercise (Stefan et al., 2004). It has been suggested that this may be explained in part by the increase of acetylcholine in the cortex in salient exercises compared to non-salient exercises (Meintzschel & Ziemann, 2006).
With regard to the functional outcomes, there was a large variety also here with regard to reported outcomes, and five of the ten chronic pain studied (De Martino, Petrini, et al., 2018; De Martino, Zandalasini, et al., 2018; Hoeger Bement et al., 2014; Mendonca et al., 2016; Schwenkreis et al., 2011) and six of the seven acute pain studies (Boudreau et al., 2007; Dancey et al., 2019; De Martino et al., 2018a; De Martino et al., 2018b; Mavromatis et al., 2017; Rittig-Rasmussen et al., 2014b), did not report specific functional outcomes. Different types of percentage performance score were reported in relation to tracking tasks (Boudreau et al., 2007; Rittig-Rasmussen et al., 2014a, 2014b) and accuracy (Baarbe et al., 2018; Mavromatis et al., 2017; Parker et al., 2017) sometimes combined with response time or speed of movement (Baarbe et al., 2018; Mavromatis et al., 2017). In general, performance improved with training regardless of presence of pain, although some performance scores were affected in pain groups compared to pain-free controls (Boudreau et al., 2007; Vallence et al., 2013).
Quality assessment
As per our Prospero protocol we carried out a risk of bias assessment utilizing two instruments. The first instrument, NOS, covers domains for selection of participants, comparability between groups, and exposure, and is the recommended instrument for case–control studies. A general finding across all studies was that the study populations were often convenience samples and therefore not truly representative of the populations under study. Furthermore, our assessment was based on the specific aim to evaluate neuroplasticity after training in the presence of pain. With regard to study design, studies without a control group can by definition only be scored on three items in the NOS and we therefore added a modified score for studies with internal control groups. Furthermore, the quality scores for some of the included studies could be higher if they had been assessed in relation to the specific aim of the respective studies. We therefore regard the results from the assessment with NOS to be of limited value in the present review, given that many studies were based on intraindividual comparisons, before and after training, and not on comparisons between groups. We also carried out an assessment with the TMS checklist which was introduced in 2012 (Chipchase et al., 2012). This instrument was developed based on a 2-round international Delphi process with 42 participants resulting in a 30-item checklist covering domains of participant characteristics, TMS protocol and analysis. The overall mean scores for the primary studies in the present review of 72 and 68% for reported and controlled factors, respectively, indicate a moderate methodological quality and are in line with other reviews utilizing this checklist. Items in the checklist that were reported to a less degree included prescribed medication and history of repetitive motor activity for participants, participants’ attention during testing, and size of unconditioned MEP in the target muscles. These findings are also in accordance with other reviews (Parker et al., 2016; Rosso & Lamy, 2018) indicating that the primary studies in the present review have a methodological quality similar to other recent systematic reviews.
Neurophysiological aspects
The main goal of many neurorehabilitation regimes is to promote neuroplasticity at the subcortical and cortical levels, such that long-lasting and beneficial alterations in motor control strategies can be achieved (Gabriel et al., 2006). Novel motor skill training, in contrast to passive assistance or repetitions of general exercise (strength training), has been associated with improvements in task performance and increased representation of the trained muscle in the motor cortex (Kothari et al., 2013; Svensson et al., 2003). At this point in time, in terms of evaluating the combined effect of pain and motor training on neuroplasticity, there was not sufficient data available that could be extracted from the primary studies and grouped according to outcome measures to carry out a meta-analysis. However, from the qualitative synthesis we can conclude that both acute pain and chronic pain may impede training-induced neuroplasticity which may have implications for motor learning and performance during rehabilitation following injury or disease. Training-induced neuroplasticity has been shown to occur rapidly and to continually evolve with more training (Koeneke et al., 2006; Svensson et al., 2003). It is therefore reasonable to assume that presence of pain may impede plasticity induced by long-term training in a similar manner to short-term training, as reported in the present review.
The results from the present review are in line with the principle that pain, both acute and chronic, is not purely a sensory process but that pain networks interact with other complex networks in cerebral structures including, but not limited to, the primary motor cortex (M1), thalamus and prefrontal cortices. Such interactions are used for example to initiate and modulate actions to avoid or reduce pain. The possible interaction between pain and the M1 can influence training-induced neuroplasticity and some of the findings in the present review may to some extent indicate the neurophysiological processes involved. Single pulse TMS measure outcomes, such as a decrease in rMT, were reported in control conditions but negated by pain. This may indicate increased excitability in a central core region of neurons in M1. On the other hand, an increase in MEPs may imply involvement of additional neurons in other regions (Hallett et al., 1999). Variations in paired pulse TMS outcomes such as ICI represent changes to the inhibitory cortical networks primarily regulated by the chief inhibitory neurotransmitter GABA (Hallett et al., 1999). Reduced activity in these inhibitory cortical networks is an indication of enhanced neuroplasticity but may be negated in the presence of pain. Furthermore, pain may also affect performance due to movement related pain or kinesiophobia directly resulting in impaired motor output (Hodges & Smeets, 2015; Hodges & Tucker, 2011). Consequently, the performed training of a specific motor skill is hampered which indirectly impedes the associated training-induced neuroplasticity.
Summary
The present study has shown that both acute pain and chronic pain may impede training-induced functional neuroplasticity manifested as decreased corticomotor excitability as defined by TMS. Overall, the findings reflect the many aspects of human neuroplasticity, that cannot be encapsulated by a single outcome measure. It should be acknowledged that other brain imaging techniques such as structural and functional magnetic resonance imaging (sMRI, fMRI), electroencephalography (EEG) and magnetoencephalography (MEG) can provide complementary information that can help identify neural correlates underlying a particular neuroplastic brain change and associated motor behaviour. This information is important for developing better rehabilitative training approaches that adequately manage pain and facilitate adaptive neuroplasticity and improved motor performance.
Data availability
Not applicable it is a systematic review, but the data can be made available by the authors if requested.
Code availability
Not applicable.
References
Akhter, R., Benson, J., Svensson, P., Nicholas, M. K., Peck, C. C., & Murray, G. M. (2014). Experimental jaw muscle pain increases pain scores and jaw movement variability in higher pain catastrophizers. Journal of Oral & Facial Pain and Headache, 28(3), 191–204. https://doi.org/10.11607/ofph.1211
Avivi-Arber, L., & Sessle, B. J. (2018). Jaw sensorimotor control in healthy adults and effects of ageing. Journal of Oral Rehabilitation, 45(1), 50–80. https://doi.org/10.1111/joor.12554
Baarbe, J. K., Yielder, P., Haavik, H., Holmes, M. W. R., & Murphy, B. A. (2018). Subclinical recurrent neck pain and its treatment impacts motor training-induced plasticity of the cerebellum and motor cortex. PLoS ONE, 13(2), e0193413. https://doi.org/10.1371/journal.pone.0193413
Bank, P. J., Peper, C. E., Marinus, J., Beek, P. J., & van Hilten, J. J. (2013). Motor consequences of experimentally induced limb pain: A systematic review. European Journal of Pain, 17(2), 145–157. https://doi.org/10.1002/j.1532-2149.2012.00186.x
Boudreau, S., Romaniello, A., Wang, K., Svensson, P., Sessle, B. J., & Arendt-Nielsen, L. (2007). The effects of intra-oral pain on motor cortex neuroplasticity associated with short-term novel tongue-protrusion training in humans. Pain, 132(1–2), 169–178. https://doi.org/10.1016/j.pain.2007.07.019
Bradnam, L., McDonnell, M., & Ridding, M. (2016). Cerebellar intermittent theta-burst stimulation and motor control training in individuals with cervical dystonia. Brain Sciences, 6(4), 56. https://doi.org/10.3390/brainsci6040056
Chang, Y. (2014). Reorganization and plastic changes of the human brain associated with skill learning and expertise. Frontiers in Human Neuroscience, 8. https://doi.org/10.3389/fnhum.2014.00035
Chipchase, L., Schabrun, S., Cohen, L., Hodges, P., Ridding, M., Rothwell, J., Taylor, J., & Ziemann, U. (2012). A checklist for assessing the methodological quality of studies using transcranial magnetic stimulation to study the motor system: An international consensus study. Clinical Neurophysiology, 123(9), 1698–1704. https://doi.org/10.1016/j.clinph.2012.05.003
Daligadu, J., Haavik, H., Yielder, P. C., Baarbe, J., & Murphy, B. (2013). Alterations in cortical and cerebellar motor processing in subclinical neck pain patients following spinal manipulation. Journal of Manipulative and Physiological Therapeutics, 36(8), 527–537. https://doi.org/10.1016/j.jmpt.2013.08.003
Dancey, E., Yielder, P., & Murphy, B. (2019). The Interactive Effect of Tonic Pain and Motor Learning on Corticospinal Excitability. Brain Sci, 9(3). https://doi.org/10.3390/brainsci9030063
De Martino, E., Petrini, L., Schabrun, S., & Graven-Nielsen, T. (2018a). Cortical Somatosensory Excitability Is Modulated in Response to Several Days of Muscle Soreness. The Journal of Pain, 19(11), 1296–1307. https://doi.org/10.1016/j.jpain.2018.05.004
De Martino, E., Zandalasini, M., Schabrun, S., Petrini, L., & Graven-Nielsen, T. (2018b). Experimental muscle hyperalgesia modulates sensorimotor cortical excitability, which is partially altered by unaccustomed exercise. Pain, 159(12), 2493–2502. https://doi.org/10.1097/j.pain.0000000000001351
Dettmers, C., Adler, T., Rzanny, R., van Schayck, R., Gaser, C., Weiss, T., Miltner, W. H., Bruckner, L., & Weiller, C. (2001). Increased excitability in the primary motor cortex and supplementary motor area in patients with phantom limb pain after upper limb amputation. Neuroscience Letters, 307(2), 109–112. https://doi.org/10.1016/s0304-3940(01)01953-x
Duchateau, J., Semmler, J. G., & Enoka, R. M. (2006). Training adaptations in the behavior of human motor units. Journal of Applied Physiology (1985), 101(6), 1766–1775. https://doi.org/10.1152/japplphysiol.00543.2006
Gabriel, D. A., Kamen, G., & Frost, G. (2006). Neural adaptations to resistive exercise: Mechanisms and recommendations for training practices. Sports Medicine (auckland, n. z.), 36(2), 133–149. https://doi.org/10.2165/00007256-200636020-00004
Gurevich, M., Kohn, P. M., & Davis, C. (1994). Exercise-induced analgesia and the role of reactivity in pain sensitivity. Journal of Sports Sciences, 12(6), 549–559. https://doi.org/10.1080/02640419408732205
Hallett, M., Chen, R., Ziemann, U., & Cohen, L. G. (1999). Reorganization in motor cortex in amputees and in normal volunteers after ischemic limb deafferentation. Electroencephalogr Clin Neurophysiol Suppl, 51, 183–187. https://www.ncbi.nlm.nih.gov/pubmed/10590950
Hellmann, D., Giannakopoulos, N. N., Blaser, R., Eberhard, L., Rues, S., & Schindler, H. J. (2011). Long-term training effects on masticatory muscles. Journal of Oral Rehabilitation, 38(12), 912–920. https://doi.org/10.1111/j.1365-2842.2011.02227.x
Hodges, P. W., & Smeets, R. J. (2015). Interaction Between Pain, Movement, and Physical Activity Short-term Benefits, Long-term Consequences, and Targets for Treatment. Clinical Journal of Pain, 31(2), 97–107. https://doi.org/10.1097/Ajp.0000000000000098
Hodges, P. W., & Tucker, K. (2011). Moving differently in pain: A new theory to explain the adaptation to pain. Pain, 152(3 Suppl), S90–S98. https://doi.org/10.1016/j.pain.2010.10.020
HoegerBement, M., Weyer, A., Yoon, T., & Hunter, S. (2014). Corticomotor Excitability During a Noxious Stimulus Before and After Exercise in Women With Fibromyalgia. Journal of Clinical Neurophysiology, 31(1), 94–98.
Ingham, D., Tucker, K. J., Tsao, H., & Hodges, P. W. (2011). The effect of pain on training-induced plasticity of the corticomotor system. European Journal of Pain, 15(10), 1028–1034. https://doi.org/10.1016/j.ejpain.2011.04.006
Kleim, J. A., & Jones, T. A. (2008). Principles of experience-dependent neural plasticity: Implications for rehabilitation after brain damage. Journal of Speech, Language, and Hearing Research, 51(1), S225-239. https://doi.org/10.1044/1092-4388(2008/018)
Koeneke, S., Lutz, K., Herwig, U., Ziemann, U., & Jäncke, L. (2006). Extensive training of elementary finger tapping movements changes the pattern of motor cortex excitability. Experimental Brain Research, 174(2), 199–209. https://doi.org/10.1007/s00221-006-0440-8
Kosek, E., Roos, E. M., Ageberg, E., & Nilsdotter, A. (2013). Increased pain sensitivity but normal function of exercise induced analgesia in hip and knee osteoarthritis–treatment effects of neuromuscular exercise and total joint replacement. Osteoarthritis Cartilage, 21(9), 1299–1307. https://doi.org/10.1016/j.joca.2013.06.019
Kothari, M., Svensson, P., Jensen, J., Kjærsgaard, A., Jeonghee, K., Nielsen, J. F., Ghovanloo, M., & Baad-Hansen, L. (2013). Training-induced cortical plasticity compared between three tongue-training paradigms. Neuroscience, 246, 1–12. https://doi.org/10.1016/j.neuroscience.2013.04.040
Krause, P., Forderreuther, S., & Straube, A. (2006). TMS motor cortical brain mapping in patients with complex regional pain syndrome type I. Clinical Neurophysiology, 117(1), 169–176. https://doi.org/10.1016/j.clinph.2005.09.012
Lohse, K. R., Wadden, K., Boyd, L. A., & Hodges, N. J. (2014). Motor skill acquisition across short and long time scales: A meta-analysis of neuroimaging data. Neuropsychologia, 59(130), 141. https://doi.org/10.1016/j.neuropsychologia.2014.05.001
Massé-Alarie, H., Louis-David, B., Preuss, R., Schneider, C. (2015). Multifidus voluntary training versus hip extension exercises in chronic low back pain: effects on clinical outcomes and underlying corticomotor function. Physiotherapy, 101e9609–e961. https://doi.org/10.1016/j.physio.2015.03.1814
Masse-Alarie, H., Beaulieu, L. D., Preuss, R., & Schneider, C. (2016). Influence of paravertebral muscles training on brain plasticity and postural control in chronic low back pain. Scandinavian Journal of Pain, 12, 74–83. https://doi.org/10.1016/j.sjpain.2016.03.005
Massé-Alarie, H., Beaulieu, L. D., Preuss, R., & Schneider, C. (2017a). The side of chronic low back pain matters: evidence from the primary motor cortex excitability and the postural adjustments of multifidi muscles. Experimental Brain Research, 235(3), 647–659. https://doi.org/10.1007/s00221-016-4834-y
Masse-Alarie, H., Beaulieu, L. D., Preuss, R., & Schneider, C. (2017b). Repetitive peripheral magnetic neurostimulation of multifidus muscles combined with motor training influences spine motor control and chronic low back pain. Clinical Neurophysiology, 128(3), 442–453. https://doi.org/10.1016/j.clinph.2016.12.020
Mavromatis, N., Neige, C., Gagne, M., Reilly, K. T., & Mercier, C. (2017). Effect of Experimental Hand Pain on Training-Induced Changes in Motor Performance and Corticospinal Excitability. Brain Sci, 7(2). https://doi.org/10.3390/brainsci7020015
McCambridge, A. B., Bradnam, L. V. (2018). S63. Cerebellar stimulation for adults with cervical dystonia: A tDCS study. Clinical Neurophysiology, 129e165. https://doi.org/10.1016/j.clinph.2018.04.423
Meintzschel, F., & Ziemann, U. (2006). Modification of practice-dependent plasticity in human motor cortex by neuromodulators. Cerebral Cortex, 16(8), 1106–1115. https://doi.org/10.1093/cercor/bhj052
Mendonca, M. E., Simis, M., Grecco, L. C., Battistella, L. R., Baptista, A. F., & Fregni, F. (2016). Transcranial Direct Current Stimulation Combined with Aerobic Exercise to Optimize Analgesic Responses in Fibromyalgia: A Randomized Placebo-Controlled Clinical Trial. Frontiers in Human Neuroscience, 10. https://doi.org/10.3389/fnhum.2016.00068
Michelini, L. C., & Stern, J. E. (2009). Exercise-induced neuronal plasticity in central autonomic networks: Role in cardiovascular control. Experimental Physiology, 94(9), 947–960. https://doi.org/10.1113/expphysiol.2009.047449
Moher, D., Liberati, A., Tetzlaff, J., Altman, D. G., & Group, P. (2009). Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Journal of Clinical Epidemiology, 62(10), 1006–1012. https://doi.org/10.1016/j.jclinepi.2009.06.005
Parker, R., Lewis, G., Rice, D., & McNair, P. (2016). Is Motor Cortical Excitability Altered in People with Chronic Pain? A Systematic Review and Meta-Analysis. Brain Stimulation, 4, 488–500. https://doi.org/10.1016/j.brs.2016.03.020
Parker, R., Lewis, G., Rice, D., & McNair, P. (2017). The Association between Corticomotor Excitability and Motor Skill Learning in People with Painful Hand Arthritis. The Clinical Journal of Pain, 33(3), 222–230. https://doi.org/10.1097/ajp.0000000000000392
Pascual-Leone, A., Nguyet, D., Cohen, L. G., Brasil-Neto, J. P., Cammarota, A., & Hallett, M. (1995). Modulation of muscle responses evoked by transcranial magnetic stimulation during the acquisition of new fine motor skills. Journal of Neurophysiology, 74(3), 1037–1045. https://doi.org/10.1152/jn.1995.74.3.1037
Pascual-Leone, A., Amedi, A., Fregni, F., & Merabet, L. B. (2005). THE PLASTIC HUMAN BRAIN CORTEX. Annual Review of Neuroscience, 28(1), 377–401. https://doi.org/10.1146/annurev.neuro.27.070203.144216
Remple, M. S., Bruneau, R. M., VandenBerg, P. M., Goertzen, C., & Kleim, J. A. (2001). Sensitivity of cortical movement representations to motor experience: evidence that skill learning but not strength training induces cortical reorganization. Behavioural Brain Research, 123(2), 133–141.
Rittig-Rasmussen, B., Kasch, H., Fuglsang-Frederiksen, A., Jensen, T. S., & Svensson, P. (2013). Specific neck training induces sustained corticomotor hyperexcitability as assessed by motor evoked potentials. Spine, 38(16), E979–E984. https://doi.org/10.1097/BRS.0b013e3182975310
Rittig-Rasmussen, B., Kasch, H., Fuglsang-Frederiksen, A., Svensson, P., & Jensen, T. S. (2014a). Effect of training on corticomotor excitability in clinical neck pain. European Journal of Pain, 18(8), 1207–1216. https://doi.org/10.1002/j.1532-2149.2014.487.x
Rittig-Rasmussen, B., Kasch, H., Fuglsang-Frederiksen, A., Svensson, P., & Jensen, T. S. (2014b). The role of neuroplasticity in experimental neck pain: A study of potential mechanisms impeding clinical outcomes of training. Manual Therapy, 19(4), 288–293. https://doi.org/10.1016/j.math.2014.04.010
Romaniello, A., Cruccu, G., McMillan, A. S., Arendt-Nielsen, L., & Svensson, P. (2000). Effect of experimental pain from trigeminal muscle and skin on motor cortex excitability in humans. Brain Research, 882(1–2), 120–127. https://doi.org/10.1016/s0006-8993(00)02856-0
Rosso, C., & Lamy, J.-C. (2018). Does Resting Motor Threshold Predict Motor Hand Recovery After Stroke? Frontiers in Neurology, 9. https://doi.org/10.3389/fneur.2018.01020
Rothwell, J. (2018). Transcranial brain stimulation: Past and future. Brain and Neuroscience Advances, 2, 239821281881807. https://doi.org/10.1177/2398212818818070
Schwenkreis, P., Voigt, M., Hasenbring, M., Tegenthoff, M., Vorgerd, M., & Kley, R. A. (2011). Central Mechanisms during Fatiguing Muscle Exercise in Muscular Dystrophy and Fibromyalgia Syndrome: A Study with Transcranial Magnetic Stimulation. Muscle & Nerve, 43(4), 479–484. https://doi.org/10.1002/mus.21920
Stang, A. (2010). Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. European Journal of Epidemiology, 25(9), 603–605. https://doi.org/10.1007/s10654-010-9491-z
Stefan, K., Wycislo, M., & Classen, J. (2004). Modulation of associative human motor cortical plasticity by attention. Journal of Neurophysiology, 92(1), 66–72. https://doi.org/10.1152/jn.00383.2003
Svensson, P., Romaniello, A., Arendt-Nielsen, L., & Sessle, B. J. (2003). Plasticity in corticomotor control of the human tongue musculature induced by tongue-task training. Experimental Brain Research, 152(1), 42–51. https://doi.org/10.1007/s00221-003-1517-2
Tsao, H., Galea, M. P., & Hodges, P. W. (2010). Driving plasticity in the motor cortex in recurrent low back pain. European Journal of Pain, 14(8), 832–839. https://doi.org/10.1016/j.ejpain.2010.01.001
Vallence, A. M., Smith, A., Tabor, A., Rolan, P. E., & Ridding, M. C. (2013). Chronic tension-type headache is associated with impaired motor learning. Cephalalgia, 33(12), 1048–1054. https://doi.org/10.1177/0333102413483932
Volz, M. S., Mendonca, M., Pinheiro, F. S., Cui, H., Santana, M., Fregni, F., & Zhan, W. (2012). Dissociation of motor task-induced cortical excitability and pain perception changes in healthy volunteers. PLoS ONE, 7(3), e34273. https://doi.org/10.1371/journal.pone.0034273
Wang, R., Ke, S., Zhang, Q., Zhou, K., Li, P., & Yang, J. (2020). Functional and structural neuroplasticity associated with second language proficiency: An MRI study of Chinese-English bilinguals. Journal of Neurolinguistics, 56, 100940. https://doi.org/10.1016/j.jneuroling.2020.100940
Wulf, G., Shea, C., & Lewthwaite, R. (2010). Motor skill learning and performance: A review of influential factors. Medical Education, 44(1), 75–84. https://doi.org/10.1111/j.1365-2923.2009.03421.x
Acknowledgements
The assistance of Mrs Martina Vall ( information specialist) is acknowledged for the electronic literature search.
Funding
Open access funding provided by Malmö University. Yuri Martins Costa acknowledges the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior, Brasil – Capes [Finance Code 001, Capes/Print 88887.468350/2019–00].
Author information
Authors and Affiliations
Contributions
NS, BHH, MK, PS: Study design; NS, BHH, MK: Literature search, article selection; NS, MK: Data extraction and analysis, NS, YMS: Quality assessment of included papers; NS, LAA: risk of bias assessment of included articles; NS, BHH: Drafted manuscript; All authors: critically reviewed manuscript and approved final version.
Corresponding author
Ethics declarations
Ethics approval, Consent to participate, Consent for publication.
Not applicable.
Conflict of Interest
None of the authors have a conflict of interest to declare.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Stanisic, N., Häggman-Henrikson, B., Kothari, M. et al. Pain’s Adverse Impact on Training-Induced Performance and Neuroplasticity: A Systematic Review. Brain Imaging and Behavior 16, 2281–2306 (2022). https://doi.org/10.1007/s11682-021-00621-6
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11682-021-00621-6