Abstract
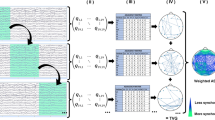
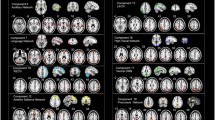
Schizophrenia is a chronic mental disorder characterized by continuous or relapsing episodes of psychosis. While previous studies have detected functional network connectivity alterations in patients with schizophrenia, and most have focused on static functional connectivity. However, brain activity is believed to change dynamically over time. Therefore, we computed dynamic functional network connectivity using the sliding window method in 38 patients with schizophrenia and 31 healthy controls. We found that patients with schizophrenia exhibited higher occurrences in the weakly and sparsely connected state (state 3) than healthy controls, positively correlated with negative symptoms. In addition, patients exhibited fewer occurrences in a strongly connected state (state 4) than healthy controls. Lastly, the dynamic functional network connectivity between the right executive-control network and the medial visual network was decreased in schizophrenia patients compared to healthy controls. Our results further prove that brain activity is dynamic, and that alterations of dynamic functional network connectivity features might be a fundamental neural mechanism in schizophrenia.



Similar content being viewed by others
Data availability
The data that support the findings of this study are available from the corresponding author upon reasonable request.
References
Abram, S. V., et al. (2017). Fronto-temporal connectivity predicts cognitive empathy deficits and experiential negative symptoms in schizophrenia. Human Brain Mapping, 38(3), 1111–1124
Abrol, A., et al., (2017). Replicability of time-varying connectivity patterns in large resting state fMRI samples. Neuroimage, 163, 160–176
Adams, R. A., et al., (2020). Impaired theta phase coupling underlies frontotemporal dysconnectivity in schizophrenia. Brain, 143(4), 1261–1277
Allen, E. A., et al. (2011). A baseline for the multivariate comparison of resting-state networks. Frontiers in Systems Neuroscience, 5, 2
Allen, E. A., et al. (2014). Tracking whole-brain connectivity dynamics in the resting state. Cerebral Cortex, 24(3), 663–676
Anhoj, S., et al. (2018). Alterations of intrinsic connectivity networks in antipsychotic-Naive first-episode schizophrenia. Schizophrenia Bulletin, 44(6), 1332–1340
Baker, J. T., et al., (2014). Disruption of cortical association networks in schizophrenia and psychotic bipolar disorder. JAMA Psychiatry, 71(2), 109–118
Barkhof, F., Haller, S., & Rombouts, S. A. (2014). Resting-state functional MR imaging: a new window to the brain. Radiology, 272(1), 29–49
Biswal, B., et al. (1995). Functional connectivity in the motor cortex of resting human brain using echo-planar MRI. Magnetic Resonance in Medicine, 34(4), 537–541
Brandl, F., et al. (2019). Specific substantial dysconnectivity in schizophrenia: a transdiagnostic multimodal meta-analysis of resting-state functional and structural magnetic resonance imaging studies. Biological Psychiatry, 85(7), 573–583
Butler, P. D., et al. (2001). Dysfunction of early-stage visual processing in schizophrenia. The American Journal of Psychiatry, 158(7), 1126–1133
Butler, P. D., et al. (2005). Early-stage visual processing and cortical amplification deficits in schizophrenia. Archives of General Psychiatry, 62(5), 495–504
Butler, P. D., Silverstein, S. M., & Dakin, S. C. (2008). Visual perception and its impairment in schizophrenia. Biological Psychiatry, 64(1), 40–47
Calhoun, V. D., et al., (2014). The chronnectome: time-varying connectivity networks as the next frontier in fMRI data discovery. Neuron, 84(2), 262–274
Carter, R., & Ffytche, D. H. (2015). On visual hallucinations and cortical networks: a trans-diagnostic review. Journal of Neurology, 262(7), 1780–1790
Chadick, J. Z., & Gazzaley, A. (2011). Differential coupling of visual cortex with default or frontal-parietal network based on goals. Nature Neuroscience, 14(7), 830–832
Charlson, F. J., et al. (2018). Global epidemiology and burden of schizophrenia: findings from the global burden of disease study 2016. Schizophrenia Bulletin, 44(6), 1195–1203
Chen, Y. (2011). Abnormal visual motion processing in schizophrenia: a review of research progress. Schizophrenia Bulletin, 37(4), 709–715
Csaszar, N., Kapocs, G., & Bokkon, I. (2019). A possible key role of vision in the development of schizophrenia. Reviews in the Neurosciences, 30(4), 359–379
d’Ambrosio, A., et al. (2020). Reduced dynamics of functional connectivity and cognitive impairment in multiple sclerosis. Multiple Sclerosis Journal, 26(4), 476–488
Damaraju, E., et al. (2014). Dynamic functional connectivity analysis reveals transient states of dysconnectivity in schizophrenia. NeuroImage: Clinical, 5, 298–308
Deng, Y., et al. (2019). Ventral and dorsal visual pathways exhibit abnormalities of static and dynamic connectivities, respectively, in patients with schizophrenia. Schizophrenia Research, 206, 103–110
Disease, G. B. D., Injury, I., & Prevalence, C. (2018). Global, regional, and national incidence, prevalence, and years lived with disability for 354 diseases and injuries for 195 countries and territories, 1990-2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet, 392(10159), 1789–1858
Dong, D., et al. (2018). Dysfunction of large-scale brain networks in schizophrenia: a meta-analysis of resting-state functional connectivity. Schizophrenia Bulletin, 44(1), 168–181
Du, Y., et al. (2016). Interaction among subsystems within default mode network diminished in schizophrenia patients: A dynamic connectivity approach. Schizophrenia Research, 170(1), 55–65
Du, Y., et al., (2018). Dynamic functional connectivity impairments in early schizophrenia and clinical high-risk for psychosis. Neuroimage, 180(Pt B), 632–645
Du, X., et al. (2019). Aberrant middle prefrontal-motor cortex connectivity mediates motor inhibitory biomarker in schizophrenia. Biological Psychiatry, 85(1), 49–59
Duan, X., et al. (2019). Effect of risperidone monotherapy on dynamic functional connectivity of insular subdivisions in treatment-Naive, first-episode schizophrenia. Schizophrenia Bulletin, 46(3), 650-660
Fiorenzato, E., et al., (2019). Dynamic functional connectivity changes associated with dementia in Parkinson’s disease. Brain, 142(9), 2860–2872
Fogelson, N., et al. (2014). The functional anatomy of schizophrenia: A dynamic causal modeling study of predictive coding. Schizophrenia Research, 158(1–3), 204–212
Gagne, A. M., Hebert, M., & Maziade, M. (2015). Revisiting visual dysfunctions in schizophrenia from the retina to the cortical cells: A manifestation of defective neurodevelopment. Progress in Neuro-Psychopharmacology & Biological Psychiatry, 62, 29–34
Gazzaley, A., et al. (2007). Functional interactions between prefrontal and visual association cortex contribute to top-down modulation of visual processing. Cerebral Cortex, 17(Suppl 1), i125-35
Himberg, J., Hyvarinen, A., & Esposito, F. (2004). Validating the independent components of neuroimaging time series via clustering and visualization. Neuroimage, 22(3), 1214–1222
Hornix, B. E., Havekes, R., & Kas, M. J. H. (2019). Multisensory cortical processing and dysfunction across the neuropsychiatric spectrum. Neuroscience & Biobehavioral Reviews, 97, 138–151
Hugdahl, K. (2009). "Hearing voices”: auditory hallucinations as failure of top-down control of bottom-up perceptual processes. Scandinavian Journal of Psychology, 50(6), 553–560
Hutchison, R. M., et al., (2013). Dynamic functional connectivity: promise, issues, and interpretations. Neuroimage, 80, 360–378
Ince, E., & Ucok, A. (2018). Relationship between persistent negative symptoms and findings of neurocognition and neuroimaging in schizophrenia. Clinical EEG and Neuroscience, 49(1), 27–35
Jimenez, A. M., et al. (2019). Linking resting-state networks and social cognition in schizophrenia and bipolar disorder. Human Brain Mapping, 40(16), 4703–4715
Kahn, R. S., et al. (2015). Schizophrenia. Nature Reviews Disease Primers, 1, 15067
Kay, S. R., Fiszbein, A., & Opler, L. A. (1987). The positive and negative syndrome scale (PANSS) for schizophrenia. Schizophrenia Bulletin, 13(2), 261–276
Kim, D., et al. (2005). Dysfunction of early-stage visual processing in schizophrenia: harmonic analysis. Schizophrenia Research, 76(1), 55–65
Kottaram, A., et al. (2018). Spatio-temporal dynamics of resting-state brain networks improve single-subject prediction of schizophrenia diagnosis. Human Brain Mapping, 39(9), 3663–3681
Kraepelin, & Emil. (1921). Dementia praecox and Paraphrenia. Journal of Nervous & Mental Disease, 54(4), 384
Kraguljac, N. V., et al. (2016). Abnormalities in large scale functional networks in unmedicated patients with schizophrenia and effects of risperidone. NeuroImage: Clinical, 10, 146–158
Kuhn, S., & Gallinat, J. (2013). Resting-state brain activity in schizophrenia and major depression: a quantitative meta-analysis. Schizophrenia Bulletin, 39(2), 358–365
Lee, J., et al. (2010). Regional brain activity during early visual perception in unaffected siblings of schizophrenia patients. Biological Psychiatry, 68(1), 78–85
Li, K., Sweeney, J. A., & Hu, X. P. (2020) Context-dependent dynamic functional connectivity alteration of lateral occipital cortex in schizophrenia. Schizophrenia Research, 220, 201-209
Ma, W. Y., et al. (2019). Dysfunctional dynamics of intra- and inter-network connectivity in dementia with lewy bodies. Frontiers in Neurology, 10, 1265
Malhi, G. S., et al. (2019). Resting-state neural network disturbances that underpin the emergence of emotional symptoms in adolescent girls: resting-state fMRI study. British Journal of Psychiatry, 215(3), 545–551
Marusak, H. A., et al. (2017). Dynamic functional connectivity of neurocognitive networks in children. Human Brain Mapping, 38(1), 97–108
Mayer, A. R., et al. (2015). An fMRI study of multimodal selective attention in schizophrenia. British Journal of Psychiatry, 207(5), 420–428
Mennigen, E., et al. (2020). State-dependent functional dysconnectivity in youth with psychosis spectrum symptoms. Schizophrenia Bulletin, 46(2), 408–421
Menon, V. (2011). Large-scale brain networks and psychopathology: a unifying triple network model. Trends in Cognitive Sciences, 15(10), 483–506
Mwansisya, T. E., et al. (2017). Task and resting-state fMRI studies in first-episode schizophrenia: A systematic review. Schizophrenia Research, 189, 9–18
O’Donoghue, S., et al. (2017). Anatomical dysconnectivity in bipolar disorder compared with schizophrenia: A selective review of structural network analyses using diffusion MRI. Journal of Affective Disorders, 209, 217–228
Pu, S., et al. (2018). Right frontotemporal cortex mediates the relationship between cognitive insight and subjective quality of life in patients with schizophrenia. Frontiers in Psychiatry, 9, 16
Rashid, B., et al. (2014). Dynamic connectivity states estimated from resting fMRI Identify differences among Schizophrenia, bipolar disorder, and healthy control subjects. Frontiers in Human Neuroscience, 8, 897
Ray, K. L., et al. (2017). Functional network changes and cognitive control in schizophrenia. NeuroImage: Clinical, 15, 161–170
Ross, L. A., et al. (2007). Impaired multisensory processing in schizophrenia: deficits in the visual enhancement of speech comprehension under noisy environmental conditions. Schizophrenia Research, 97(1–3), 173–183
Rotarska-Jagiela, A., et al. (2010). Resting-state functional network correlates of psychotic symptoms in schizophrenia. Schizophrenia Research, 117(1), 21–30
Sakoglu, U., et al., (2010). A method for evaluating dynamic functional network connectivity and task-modulation: application to schizophrenia. MAGMA, 23(5-6), 351–366
Sandsten, K. E., et al. (2020). Altered self-recognition in patients with schizophrenia. Schizophrenia Research, 218, 116-123
Schultz, S. K., & Andreasen, N. C. (1999). Schizophrenia. The Lancet, 353(9162), 1425–1430
Seeley, W. W., et al. (2007). Dissociable intrinsic connectivity networks for salience processing and executive control. The Journal of Neuroscience, 27(9), 2349–2356
Silverstein, S. M., & Lai, A. (2021). The phenomenology and neurobiology of visual distortions and hallucinations in schizophrenia: an update. Frontiers in Psychiatry, 12, 684720
Shen, H. H. (2015). Core Concept: Resting-state connectivity. Proceedings of the National Academy of Sciences of the United States of America, 112(46), 14115–14116
Skudlarski, P., et al. (2010). Brain connectivity is not only lower but different in schizophrenia: a combined anatomical and functional approach. Biological Psychiatry, 68(1), 61–69
Sridharan, D., Levitin, D. J., & Menon, V. (2008). A critical role for the right fronto-insular cortex in switching between central-executive and default-mode networks. Proceedings of the National Academy of Sciences of the United States of America, 105(34), 12569–12574
Stekelenburg, J. J., et al. (2013). Deficient multisensory integration in schizophrenia: an event-related potential study. Schizophrenia Research, 147(2–3), 253–261
Stevenson, R. A., et al. (2017). The associations between multisensory temporal processing and symptoms of schizophrenia. Schizophrenia Research, 179, 97–103
Wang, X., et al. (2014). Disrupted resting-state functional connectivity in minimally treated chronic schizophrenia. Schizophrenia Research, 156(2–3), 150–156
Wang, J., et al. (2015). GRETNA: a graph theoretical network analysis toolbox for imaging connectomics. Frontiers in Human Neuroscience, 9, 386
Wu, X. J., et al. (2017). Functional network connectivity alterations in schizophrenia and depression. Psychiatry Research: Neuroimaging, 263, 113–120
Wu, X., et al. (2019). Personality traits are related with dynamic functional connectivity in major depression disorder: A resting-state analysis. Journal of Affective Disorders, 245, 1032–1042
Zhang, M., et al. (2020). Abnormal amygdala subregional-sensorimotor connectivity correlates with positive symptom in schizophrenia. NeuroImage: Clinical, 26, 102218
Zhi, D., et al. (2018). Aberrant dynamic functional network connectivity and graph properties in major depressive disorder. Frontiers in Psychiatry, 9, 339
Funding
This work was funded by National Key R&D Program of China (2016YFC1306800), Tianjin Science and Technology Plan Projects (2017ZXMFSY00070), and Key Discipline Project for Psychiatry of Tianjin.
Author information
Authors and Affiliations
Contributions
Weiliang Yang designed the study, writing of the manuscript. Xuexin Xu, Chunxiang Wang and Yongying Cheng: recruitment of patients. Yan Li and Shuli Xu: processed the fMRI data, performed the analysis. Jie Li: reviewed and revised the article.
All authors contributed to and have approved the fnal manuscript.
Corresponding author
Ethics declarations
Conflicts of interest
All authors of this paper have no conflicts of interest to declare.
Ethics approval
The study was approved by the Ethics Committee of Tianjin Anding Hospital.
Consent to participate and for publication
All participants were provided a written informed consent to participate in this study.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
ESM 1
(DOCX 24.1 KB)
Rights and permissions
About this article
Cite this article
Yang, W., Xu, X., Wang, C. et al. Alterations of dynamic functional connectivity between visual and executive-control networks in schizophrenia. Brain Imaging and Behavior 16, 1294–1302 (2022). https://doi.org/10.1007/s11682-021-00592-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11682-021-00592-8