Abstract
Few studies have reported on brain functional differences between healthy individuals with auditory verbal hallucinations (Hi-AVH) with and without insight, so we designed a study to address this knowledge gap. We enrolled 12 Hi-AVH with insight, 15 Hi-AVH without insight, and 15 AVH-free controls (Healthy controls). Global functional connectivity density (gFCD) mapping was used to estimate brain networks. We found that the most common alterations in both Hi-AVH groups were increased gFCD in superior parietal lobule and superior temporal gyrus. We also found that distinct brain functional patterns of Hi-AVH without insight comprised lower gFCD in the frontal lobe oculomotor area, dorsolateral prefrontal cortex, supramarginal gyrus, primary auditory cortex, sensorimotor cortex, ventral anterior, and posterior cingulate Our pilot findings support the hypothesis that abnormal reciprocal action in the circuits for processing perception, memory, language, and attentional control may be pathological features of auditory verbal hallucinations.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
According to the strictest criteria proposed by Johns (“Did you at any time hear voices saying quite a few words or sentences when there was no one around that might account for it?”), 0.7% of the general population have experienced auditory verbal hallucinations (AVH) (Beavan et al. 2011; Johns et al. 2004). Of those who have experienced AVH, ones who “did not have clinically defined delusions, disorganization, or negative or catatonic symptoms, nor did they meet criteria for cluster A personality disorder” (Sommer et al. 2010) can be defined as healthy individuals with AVH (Hi-AVH). Within this population, AVH is a risk factor for psychosis or other mental disorders requiring medical care (Daalman et al. 2016).
In cases of psychosis, insight, or awareness of the disorder and its consequences, is often a strong predictor of prognosis. Previous studies of AVH in patients with schizophrenia found that lack of insight is associated with AVH deterioration and treatment difficulties. On the other hand, good insight may help alleviate the symptoms of AVH in patients with schizophrenia (Chang et al. 2018; Xavier et al. 2018; Waters 2012; Pijnenborg et al. 2013; Emami et al. 2016). Studies have reported that insight is associated with structural and functional brain alterations and that these brain alterations are in turn associated with treatment outcomes (Buchy et al. 2015; Sapara et al. 2007; Buchy et al. 2011; Bose et al. 2014). Together these studies suggest that insight and AVH are associated with distinct patterns of brain structure and function, and point specifically to regions in the temporal, frontal, and parietal lobules.
Given that Hi-AVH is a risk factor for psychosis and other mental disorders requiring medical intervention (Sommer et al. 2010; Daalman et al. 2016), investigation into the functional brain patterns involved may provide critical information to better understand the pathological features of AVH and the role of insight in disease progression. However, it is important to avoid confounding factors such as therapeutic agents and other psychotic symptoms in order to determine the precise brain patterns associated with AVH. A voxel-wise, data-driven method known as global functional connectivity density (gFCD) mapping is widely used to test the density distribution of whole-brain resting-state functional connectivity. gFCD is thought to reflect the brain’s ability to process information (Qin et al. 2015) and can be considered a biomarker for quantitative state changes in glucose metabolism (Thompson et al. 2016). gFCD has been used to investigate brain functional connectivity alterations in several mental disorders (Cohen et al. 2018; Huang et al. 2017), including schizophrenia with AVH (Zhuo et al. 2019). Hence, in the present study we adopted gFCD to investigate the common and distinct functional brain patterns in Hi-AVH with and without insight.
Methods
Samples
This study was approved by the ethics committee of Wenzhou Seventh People’s Hospital. All subjects were provided with detailed information on study process and purpose, and all subjects gave their informed consent. A total of 50 Hi-AVH and 50 AVH-free controls were enrolled to participate in the study by advertising in the community from July 2017 to December 2018. Hi-AVH inclusion criteria were as follows: 1) have AVH satisfying the proposed criteria by Johns “Did you at any time hear voices saying quite a few words or sentences when there was no one around that might account for it?”; 2) no other psychotic symptoms as determined by structured clinical interviews using the DSM-IV Axis I Disorders-Patient Edition (SCID-I/P) conducted by two senior psychiatrists with more than 10 years of experience; 3) IQ >80. The exclusion criteria were as follows: 1) other psychotic or affective disorders, mental retardation, alcohol dependence, drug dependence, organic brain lesions, or physical and neurological diseases; 2) history of unconsciousness for more than 5 min caused by any reason; 3) contraindications for MRI examination; 4) claustrophobia; 5) IQ < 80. All subjects were right-handed. Controls were distinguished by a professional psychiatrist using the SCID non-patient version.
Assessment of AVH severity
In this study, AVH severity was assessed using the auditory hallucinations rating scale (AHRS) (Hoffman et al. 2000). All subjects were assessed by a trained psychiatrist for affective and psychotic DSM-IV axis I pathologies using the Comprehensive Assessment of Symptoms and History (CASH) (Larøi et al. 2004). Global functioning was estimated using the Global Assessment of Functioning (GAF) scale (Andreasen et al. 1992), on which normal, healthy adults typically score above 90. To assess axis II pathologies, SCID-II was added to the psychiatric screening 6 months after the start of the study (First et al. 1995). The presence of psychiatric disorders in family members of the participants were quantified using the Family Interview for Genetic Studies (Takahashi et al. 2005). Urine samples were obtained to screen for drugs of abuse (including cannabis, amphetamine, cocaine, methadone, and heroin), the presence of which were grounds for exclusion from the study. Individuals with psychotic DSM-IV axis I and axis II pathologies or a family history of psychiatric disorders were also excluded. The Insight and Treatment Attitudes Questionnaire (ITAQ) was used to distinguish “with insight” vs. “without insight”. An ITAQ score of 22 was defined as having full insight, while a score of 0 was defined as having no insight. In this study, to maximize the distinction between groups, we selected only those patients with full or no insight, and discarded individuals with partial insight.
MRI data acquisition
Functional magnetic resonance imaging (fMRI) was performed on a 3 T GE Discovery MR750 scanner (General Electric, Milwaukee, WI, USA) equipped with an eight-channel phased-array head coil. The participants were instructed to lie down in a supine position and to rest without falling asleep during the scan. Whole-brain resting-state fMRI data depicting blood oxygen level-dependent signals were obtained using a gradient-echo echo-planar imaging sequence with the following parameters: repetition time (TR) = 2000 msec; echo time (TE) = 45 msec; slices = 32; slice thickness = 4 mm; gap = 0.5 mm; field of view (FOV) = 220 × 220; matrix size = 64 × 64; and flip angle (FA) = 90°. All scans were acquired by parallel imaging using the sensitivity encoding (SENSE) technique with a SENSE factor of 2. Structural images were obtained with a high-resolution 3D Turbo-Fast Echo T1WI sequence with the following parameters: 188 slices, TR/TE = 8.2/3.2, slice thickness = 1 mm, no gap, FA = 12°, matrix size = 256 × 256, FOV = 256 × 256.
Data preprocessing
SPM8 software was used to process the resting-state fMRI scans (http://www.fil.ion.ucl.ac.uk/spm). To allow for imaging unit stabilization and subject familiarization, the first 10 volumes of each scan were discarded. The remaining volumes were corrected for slice-timing and motion artifacts. Head translation movement for all participants was less than 2 mm, and rotation was less than 2°. Covariates, including head motion, white matter signal, and cerebrospinal fluid signal, were regressed out from the time series of every voxel. The Friston 24-parameter model was used to regress out head motion effects. Data were regressed out if the framewise displacement of a specific volume was >0.5. The datasets were filtered with band pass frequencies ranging from 0.01 to 0.08 Hz. Individual structural images were co-registered to the mean functional image, and the transformed structural images were co-registered to Montreal Neurological Institute (MNI) space using a linear registration. Motion-corrected functional volumes were spatially normalized to MNI space using parameters estimated during the linear co-registration. Finally, the functional images were re-sampled into 3 mm cubic voxels for further analysis.
Calculation of gFCD
The functional connectivity of each voxel was calculated using an in-house Linux script as previously reported; briefly, Pearson’s linear correlation was applied with a correlation coefficient threshold of R > 0.6 (Tomasi et al., 2010; Zou et al., 2016). The gFCD calculations were limited to those voxels within the cerebral gray matter mask, and the gFCD at any given voxel (×0) was calculated as the total number of functional connections, denoted as k (×0), between ×0 and all other voxels using a growth algorithm, which was repeated for all of the ×0 voxels. Next, gFCD was divided by the mean value of each voxel in the brain to increase the normality of the distribution. The FCD maps were spatially smoothed with a 6 × 6 × 6 mm3 Gaussian kernel to minimize differences in brain anatomy between subjects.
Statistical analysis
Group differences in gFCD among the three groups were tested using a voxel-wise one-way analysis of covariance (ANCOVA) with age, gender, and education level as covariates, followed by post hoc intergroup comparisons. The post hoc intergroup comparisons were conducted within a mask showing gFCD differences from the ANCOVA analysis, and T value was used to aid in contrast indicators (Tomasi et al., et al., 2010). Multiple comparisons were corrected using the family wise error (FWE) method with a significance threshold of P<0.05.
To investigate the relationship between gFCD and total AHRS scores, a voxel-wise multiple regression analysis was conducted in the Hi-AVH group within regions showing significant gFCD differences compared with the other two groups. Gender, age, and education level were considered as nuisance covariates. Multiple comparisons were corrected using the FWE method with a significance threshold of P<0.05. To investigate the relationship between gFCD and ITAQ scores, a voxel-wise multiple regression analysis was conducted in the Hi-AVH with insight group within regions showing significant gFCD differences compared with the other two groups. Gender, age, GAF score, and education level were considered as nuisance covariates. Multiple comparisons were corrected using the FWE method with a significance threshold of P<0.05.
Differences in the demographic measurements among these three groups were examined using Chi-square test (gender) or one-way ANOVA (age and education level). Differences in the social demographic information between the two Hi-AVH groups were tested using a two-sample t-test.
Results
Demographic and clinical characteristics
Sixteen Hi-AVH with insight, 17 Hi-AVH without insight, and 20 healthy controls were enrolled in the analysis. These three groups were matched in terms of gender (X2 = 0.198, P = 0.911), age (F = 0.209, P = 0.903), and education level (F = 0.452, P = 0.520). There were no significant differences in AVH severity (t = 2.175, P = 0.423) or duration (t = 1.010, P = 0.211) between the Hi-AVH groups with and without insight. There was, however, a significant difference in GAF score between groups (P < 0.05; Table 1).
gFCD differences
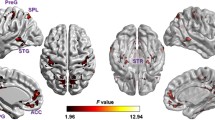
Compared to AVH-free controls, the Hi-AVH group without insight showed higher gFCD located mainly in the postcentral gyrus, superior parietal lobule, superior temporal gyrus, and temporal pole, and lower gFCD located in the superior frontal gyrus and lingual gyrus (Fig. 1a). Compared to AVH-free controls, the Hi-AVH group with insight showed higher gFCD in the inferior frontal gyrus, superior parietal lobule, superior temporal gyrus, and angular gyrus, and lower gFCD in frontal pole (Fig. 1b). The most common gFCD alterations between Hi-AVH groups and controls involved the superior parietal lobule and superior temporal gyrus. We therefore considered these alterations to be common brain functional pathological features of AVH. Differences in gFCD between the two Hi-AVH groups were located in the postcentral gyrus, temporal pole, superior frontal gyrus, and lingual gyrus. We therefore defined these regions as insight-related (FWE corrected at voxel level, P<0.05).
Compared to Hi-AVH with insight, Hi-AVH without insight had lower gFCD in the frontal lobe oculomotor area, dorsolateral prefrontal cortex, supramarginal gyrus, primary auditory cortex, sensorimotor cortex, ventral anterior, and posterior cingulate (Fig. 1c). We defined these alterations as distinctive features of Hi-AVH without insight.
Correlation analysis
We did not observe any significant correlation between gFCD and AVH severity in either Hi-AVH group.
Discussion
In this pilot study, we aimed to detect the common and distinct intrinsic functional features in Hi-AVH with and without insight. We observed that gFCD alterations in the Hi-AVH group without insight were more severe than in those with insight.
Brain regions that were altered in both Hi-AVH groups were ones involved in perception, memory, language, and central executive control. These findings support the “Resting-State Hypothesis of AVH III: Reduced Rest-external Stimulus Interaction in the Auditory Cortex” (Northoff G, et al., 2014; Branislava Ćurčić-Blake et al. 2017; Ben et al. 2016; Bohlken et al. 2017; Bose et al. 2017; Hugdahl and Sommer 2018; Kubera et al. 2019; NIMH 1992). This hypothesis postulates that abnormal strong rest-rest interactions in auditory cortex are perceived as externally, rather than internally, derived voices.
Our findings also support the hypothesis that abnormal reciprocal action of the perception, memory, language, and central executive networks can contribute to AVH (Alderson-Day et al. 2015; Hugdahl 2015; Ćurčić-Blake et al. 2017; Hugdahl 2017; Alderson-Day et al. 2016). Compared to the Hi-AVH group with insight, the Hi-AVH group without insight showed greater disturbance within these networks, and also within components of mood regulation circuitry. Previous studies have reported that functional alterations in these circuits are not only related to AVH but also to insight (Webb et al. 2016; Korovkin et al. 2018; Hill and Kemp 2018; Belvederi Murri et al. 2016; Tik et al. 2018; Sapara et al. 2016).
We did not observe any significant relationship between ARHS severity and alterations in gFCD, although such a relationship has been reported by others (Lefort-Besnard et al. 2018; Torres et al. 2016; Torres et al. 2016; Wei et al. 2016). It is possible that brain alterations as measured by gFCD may only offer a qualitative index, not a quantitative one, of AVH. Further studies are needed to clarify this point.
There are several limitations to this pilot study. First, the small sample size limits the generalizability of our findings. Second, since it was only a cross-sectional study, prospective longitudinal studies will be necessary to more fully describe the relationship between functional brain alterations and clinical symptoms and to explore early intervention strategies.
Conclusion
To the best of our knowledge, this is the first study focusing on alterations in functional connectivity density in Hi-AVH with and without insight. The salient findings of this pilot study were that there are indeed common and distinct brain functional alterations related to insight. Moreover, these findings support the hypothesis that abnormal reciprocal action of perception, memory, language, and attentional-control circuits contributes to the pathological features of AVH. Although some limitations existed, our pilot study nevertheless provided important clues to guide further studies.
References
Alderson-Day, B., Diederen, K., Fernyhough, C., Ford, J. M., Horga, G., Margulies, D. S., McCarthy-Jones, S., Northoff, G., Shine, J. M., Turner, J., van de Ven, V., van Lutterveld, R., Waters, F., & Jardri, R. (2016). Auditory hallucinations and the Brain’s resting-state networks: Findings and methodological observations. Schizophrenia Bulletin, 42, 1110–1123.
Alderson-Day, B., McCarthy-Jones, S., & Fernyhough, C. (2015). Hearing voices in the resting brain: A review of intrinsic functional connectivity research on auditory verbal hallucinations. Neuroscience and Biobehavioral Reviews, 55, 78–87.
Andreasen, N. C., Flaum, M., & Arndt, S. (1992). The comprehensive assessment of symptoms and history (CASH). An instrument for assessing diagnosis and psychopathology. Archives of General Psychiatry, 49, 615–623.
Beavan, V., Read, J., & Cartwright, C. (2011). The prevalence of voice hearers in the general population: A literature review. Journal of Mental Health, 20, 281–292.
Ben, A., Kelly, D., & Charles, F. (2016). Auditory hallucinations and the Brain’s resting-state networks: Findings and methodological observations. Schizophrenia Bulletin, 42(5), 1110–1123.
Belvederi Murri, M., Amore, M., Calcagno, P., Respino, M., Marozzi, V., Masotti, M., Bugliani, M., Innamorati, M., Pompili, M., Galderisi, S., & Maj, M. (2016). The “insight paradox” in schizophrenia: Magnitude, moderators and mediators of the association between insight and depression. Schizophrenia Bulletin, 42, 1225–1233.
Bohlken MM, Hugadahl K et. al., Auditory verbal hallucinations: Neuroimaging and treatment. Psychological Medicine, 2017.
Bose, A., Shivakumar, V., Agarwal, S. M., Kalmady, S. V., Shenoy, S., Sreeraj, V. S., Narayanaswamy, J. C., & Venkatasubramanian, G. (2017). Efficacy of fronto-temporal transcranial direct current stimulation for refractory auditory verbal hallucinations in schizophrenia: A randomized, double-blind, sham-controlled study. Schizophrenia Research, 195, 475–480.
Bose, A., Shivakumar, V., Narayanaswamy, J. C., Nawani, H., Subramaniam, A., Agarwal, S. M., Chhabra, H., Kalmady, S. V., & Venkatasubramanian, G. (2014). Insight facilitation with add-on tdcs in schizophrenia. Schizophrenia Research, 156, 63–65.
Buchy, L., Ad-Dab’bagh, Y., Malla, A., Lepage, C., Bodnar, M., Joober, R., Sergerie, K., Evans, A., & Lepage, M. (2011). Cortical thickness is associated with poor insight in first-episode psychosis. Journal of Psychiatric Research, 45, 781–787.
Buchy, L., Hawco, C., Joober, R., Malla, A., & Lepage, M. (2015). Cognitive insight in first-episode schizophrenia: Further evidence for a role of the ventrolateral prefrontal cortex. Schizophrenia Research, 166, 65–68.
Chang, C. C., Tzeng, N. S., Chao, C. Y., Yeh, C. B., & Chang, H. A. (2018). The effects of add-on Fronto-temporal transcranial direct current stimulation (tDCS) on auditory verbal hallucinations, other psychopathological symptoms, and insight in schizophrenia: A randomized, double-blind, sham-controlled trial. The International Journal of Neuropsychopharmacology, 21, 979–987.
Cohen, A. D., Tomasi, D., Shokri-Kojori, E., Nencka, A. S., & Wang, Y. (2018). Functional connectivity density mapping: Comparing multiband and conventional EPI protocols. Brain Imaging and Behavior, 12, 848–859.
Ćurčić-Blake, B., Ford, J. M., Hubl, D., Orlov, N. D., Sommer, I. E., Waters, F., Allen, P., Jardri, R., Woodruff, P. W., David, O., Mulert, C., Woodward, T. S., & Aleman, A. (2017). Interaction of language, auditory and memory brain networks in auditory verbal hallucinations. Progress in Neurobiology, 148, 1–20.
Daalman, K., Diederen, K. M., Hoekema, L., van Lutterveld, R., & Sommer, I. E. (2016). Five year follow-up of non-psychotic adults with frequent auditory verbal hallucinations: Are they still healthy? Psychological Medicine, 46, 1897–1907.
Emami, S., Guimond, S., Chakravarty, M. M., & Lepage, M. (2016). Cortical thickness and low insight into symptoms in enduring schizophrenia. Schizophrenia Research, 170, 66–72.
First, M. B., Spitzer, R. L., & Gibbon, M. (1995). Structured clinical interview for personality disorder (SCID-II): Multi-site test-retestreliability study. J Pers Disorder, 9, 92–104.
Hill, G., & Kemp, S. M. (2018). Connect 4: A novel paradigm to elicit positive and negative insight and search problem solving. Frontiers in Psychology, 9, 1755.
Hoffman, R. E., Boutros, N. N., Hu, S., Berman, R. M., Krystal, J. H., & Charney, D. S. (2000). Transcranial magnetic stimulation and auditory hallucinations in schizophrenia. Lancet, 355, 1073–1075.
Huang, H., Jiang, Y., Xia, M., Tang, Y., Zhang, T., Cui, H., Wang, J., Li, Y., Xu, L., Curtin, A., Sheng, J., Jia, Y., Yao, D., Li, C., & Luo, C. (2017). Wang J (2017) increased resting-state global functional connectivity density of default mode network in schizophrenia subjects treated with electroconvulsive therapy. Schizophrenia Research. https://doi.org/10.1016/j.schres.2017.10.044.
Hugdahl, K. (2015). Auditory hallucinations: A review of the ERC “VOICE” project world J. Psychiatry, 5, 193–209.
Hugdahl, K. (2017). Auditory hallucinations as translational psychiatry: Evidence from magnetic resonance imaging. Balkan Medical Journal, 34, 504–513.
Hugdahl, K., & Sommer, I. E. (2018). Auditory verbal hallucinations in schizophrenia from a levels of explanation perspective. Schizophrenia Bulletin, 44, 234–241.
Johns, L. C., Cannon, M., Singleton, N., Murray, R. M., Farrell, M., Brugha, T., Bebbington, P., Jenkins, R., & Meltzer, H. (2004). Prevalence and correlates of self-reported psychotic symptoms in the British population. The British Journal of Psychiatry, 185, 298–305.
Korovkin, S., Vladimirov, I., Chistopolskaia, A., & Savinova, A. (2018). How working memory provides representational change during insight problem solving. Frontiers in Psychology, 9, 1864.
Kubera, K. M., Rashidi, M., Schmitgen, M. M., Barth, A., Hirjak, D., Sambataro, F., Calhoun, V. D., & Wolf, R. C. (2019). Structure/function interrelationships in patients with schizophrenia who have persistent auditory verbal hallucinations: A multimodal MRI study using parallel ICA. Progress in Neuro-Psychopharmacology & Biological Psychiatry, 93, 114–121.
Larøi, F., Marczewski, P., & Van der Linden, M. (2004). Further evidence of the multi-dimensionality of hallucinatory predisposition: Factor structure of a modified version of the Launay-Slade Hallucina-tions scale in a normal sample. European Psychiatry, 19, 15–20.
Lefort-Besnard, J., Bassett, D. S., Smallwood, J., Margulies, D. S., Derntl, B., Gruber, O., Aleman, A., Jardri, R., Varoquaux, G., Thirion, B., Eickhoff, S. B., & Bzdok, D. (2018). Different shades of default mode disturbance in schizophrenia: Subnodal covariance estimation in structure and function. Human Brain Mapping, 39, 644–661.
NIMH. (1992). Genetics initiative: Family interview for genetic studies (FIGS). National Institute of Mental Health: Rockville.
Northoff, G. Are Auditory Hallucinations Related to the Brain's Resting State Activity? A 'Neurophenomenal Resting State Hypothesis'.(2014). Clin Psychopharmacol Neurosci, 12:189–195.
Pijnenborg, G. H., van Donkersgoed, R. J., David, A. S., & Aleman, A. (2013). Changes in insight during treatment for psychotic disorders: A meta-analysis. Schizophr Res, 144, 109–117.
Qin, W., Xuan, Y., Liu, Y., Jiang, T., & Yu, C. (2015). Functional connectivity density in congenitally and late blind subjects. Cerebral Cortex, 25, 2507–2516.
Sapara, A., Cooke, M., Fannon, D., Francis, A., Buchanan, R. W., Anilkumar, A. P., Barkataki, I., Aasen, I., Kuipers, E., & Kumari, V. (2007). Prefrontal cortex and insight in schizophrenia: A volumetric MRI study. Schizophrenia Research, 89, 22–34.
Sapara, A., Ffytche, D. H., Cooke, M. A., Williams, S. C., & Kumari, V. (2016). Voxel-based magnetic resonance imaging investigation of poor and preserved clinical insight in people with schizophrenia. World J Psychiatry, 6, 311–321.
Sommer, I. E., Daalman, K., Rietkerk, T., Diederen, K. M., Bakker, S., Wijkstra, J., & Boks, M. P. (2010). Healthy individuals with auditory verbal hallucinations; who are they? Psychiatric assessments of a selected sample of 103 subjects. Schizophrenia Bulletin, 36, 633–641.
Takahashi, S., Faraone, S.V., Lasky-Su, J., Tsuang, M.T.(2005). Genome-wide scan of homogeneous subtypes of NIMH genetics initiative schizophrenia families. Psychiatry Res, 133, 111–122.
Thompson, G. J., Riedl, V., Grimmer, T., Drzezga, A., Herman, P., & Hyder, F. (2016). The whole-brain "global" signal from resting state fMRI as a potential biomarker of quantitative state changes in glucose metabolism. Brain Connectivity, 6, 435–447.
Tik, M., Sladky, R., Luft, C. D., Willinger, D., Hoffmann, A., Banissy, M. J., Bhattacharya, J., & Windischberger, C. (2018). Ultra-high-field fMRI insights on insight: Neural correlates of the aha!-moment. Human Brain Mapping, 39, 3241–3252.
Tomasi, D., & Volkow, N. D. (2010). Functional connectivity density mapping. Proceedings of the National Academy of Sciences of the United States of America, 107, 9885–9890.
Torres, U. S., Duran, F. L., Schaufelberger, M. S., Crippa, J. A., Louzã, M. R., Sallet, P. C., Kanegusuku, C. Y., Elkis, H., Gattaz, W. F., Bassitt, D. P., Zuardi, A. W., Hallak, J. E., Leite, C. C., Castro, C. C., Santos, A. C., Murray, R. M., & Busatto, G. F. (2016). Patterns of regional gray matter loss at different stages of schizophrenia: A multisite, cross-sectional VBM study in first-episode and chronic illness. Neuroimage Clin, 12, 1–15.
Waters, F. (2012). Multidisciplinary approaches to understanding auditory hallucinations in schizophrenia and nonschizophrenia populations: The international consortium on hallucination research. Schizophrenia Bulletin, 38, 693–694.
Webb, M. E., Little, D. R., & Cropper, S. J. (2016). Insight is not in the problem: Investigating insight in problem solving across task types. Frontiers in Psychology, 7, 1424. https://doi.org/10.3389/fpsyg.2016.01424.
Wei, Y. Y., Wang, J. J., Yan, C., Li, Z. Q., Pan, X., Cui, Y., Su, T., Liu, T. S., & Tang, Y. X. (2016). Correlation between brain activation changes and cognitive improvement following cognitive remediation therapy in schizophrenia: An activation likelihood estimation meta-analysis. Chinese Medical Journal, 129, 578–585.
Xavier, R. M., Pan, W., Dungan, J. R., Keefe, R. S., & Vorderstrasse, A. (2018). Unraveling interrelationships among psychopathology symptoms, cognitive domains and insight dimensions in chronic schizophrenia. Schizophrenia Research, 193, 83–90.
Zhuo, C., Xu, Y., Zhang, L., Jing, R., & Zhou, C. (2019). The effect of dopamine antagonist treatment on auditory verbal hallucinations in healthy individuals is clearly influenced by COMT genotype and accompanied by corresponding brain structural and functional alterations: An artificially controlled pilot study. Frontiers in Genetics, 10, 92. https://doi.org/10.3389/fgene.2019.00092.
Zou, L., Guo, Q., Xu, Y., Yang, B., Jiao, Z., Xiang, J.(2016).Functional connectivity analysis of the neural bases of emotion regulation: A comparison of independent component method with density-based k-means clustering method. Technol Health Care, 24 Suppl 2:S817–825.
Acknowledgements
This work was supported by grants from the National Natural Science Foundation of China (81871052 to C.Z.), the Key Projects of the Natural Science Foundation of Tianjin, China (17JCZDJC35700 to C.Z.), the Tianjin Health Bureau Foundation (2014KR02 to C.Z.), the Zhejiang Public Welfare Fund Project (LGF18H090002 to D.J), and the key project of the Wenzhou Science and Technology Bureau (ZS2017011 to X.L).
Author information
Authors and Affiliations
Contributions
CZ conceived the study. CZ, FJ, XL, HT, CC, collected the data. LW, YX, WW, DJ analyzed the data. CZ drafted the first manuscript. All the authors critically revised the manuscript for intellectual content and approved the final version.
Corresponding author
Ethics declarations
Conflict of interest
The authors have no conflicts of interest to disclose.
Ethical approval
This study was approved by the ethics committee of Wenzhou Seventh People’s Hospital.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Sponsor’s role
The sponsors did not participate in the design, methods, subject recruitment, data collections, analysis or preparation of this manuscript.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Zhuo, C., Ji, F., Lin, X. et al. Without insight accompanied with deteriorated brain functional alterations in healthy individuals with auditory verbal hallucinations: a pilot study. Brain Imaging and Behavior 14, 2553–2558 (2020). https://doi.org/10.1007/s11682-019-00207-3
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11682-019-00207-3