Abstract
The insular cortex is proposed to function as a central brain hub characterized by wide-spread connections and diverse functional roles. As a result, its centrality in the brain confers high metabolic demands predisposing it to dysfunction in disease. However, the functional profile and vulnerability to degeneration varies across the insular sub-regions. The aim of this systematic review and meta-analysis is to summarize and quantitatively analyze the relationship between insular cortex sub-regional atrophy, studied by voxel based morphometry, with cognitive and neuropsychiatric deficits in frontotemporal dementia (FTD), Alzheimer’s disease (AD), Parkinson’s disease (PD), and dementia with Lewy bodies (DLB). We systematically searched through Pubmed and Embase and identified 519 studies that fit our criteria. A total of 41 studies (n = 2261 subjects) fulfilled the inclusion criteria for the meta-analysis. The peak insular coordinates were pooled and analyzed using Anatomic Likelihood Estimation. Our results showed greater left anterior insular cortex atrophy in FTD whereas the right anterior dorsal insular cortex showed larger clusters of atrophy in AD and PD/DLB. Yet contrast analyses did not reveal significant differences between disease groups. Functional analysis showed that left anterior insular cortex atrophy is associated with speech, emotion, and affective-cognitive deficits, and right dorsal atrophy with perception and cognitive deficits. In conclusion, insular sub-regional atrophy, particularly the anterior dorsal region, may contribute to cognitive and neuropsychiatric deficits in neurodegeneration. Our results support anterior insular cortex vulnerability and convey the differential involvement of the insular sub-regions in functional deficits in neurodegenerative diseases.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The insular cortex generally sub-serves the integration of autonomic, viscero-sensory, and interoceptive functions and plays a role in cognition, decision-making and processing of emotions (Augustine 1996; Flynn 1999; Christopher et al. 2014; Uddin et al. 2017). Through specialized cells in the anterior insula called von Economo neurons (VENs), it is hypothesized to play a role in social awareness and consciousness (Craig 2009; Allman et al. 2011; Evrard et al. 2012). The diversity of functions played by the insular cortex is paralleled to its widespread connectivity with functionally different brain regions. The anterior ventral insula has preferential connections to limbic regions and is involved in emotional processing and consciousness. Whereas, the posterior insula has connections with neocortical somatosensory regions, and plays a role in interoception. In addition, the anterior insular cortex encompasses a transitional dorsal area connected to multiple limbic and neocortical brain regions, which plays a role in cognition and decision making (Mesulam and Mufson 1982; Augustine 1996; Uddin et al. 2017).
The insular cortex has shown grey matter abnormalities across 26 neurodegenerative and neuropsychiatric diseases (Crossley et al. 2014). Its atrophy may contribute to an array of cognitive and neuropsychiatric deficits depending on the sub-regions involved. In frontotemporal dementia (FTD), a family of disorders with behavioral, socio-emotional, and language deficits, the anterior part of the insula appears to be selectively vulnerable to degeneration (Seeley 2010). Moreover, a multi-center study showed that insular cortex atrophy contributed to a high diagnostic accuracy, using MRI, in diagnosing the behavioral variant of FTD (Meyer et al. 2017). Similarly, atrophy of the insular cortex is associated with the development of neuropsychiatric deficits such as agitation, apathy, and psychosis in patients suffering from Alzheimer’s disease (AD) (Rosenberg et al. 2015). In Parkinson’s disease (PD), voxel based morphometry (VBM) studies reported insular cortex atrophy particularly in PD patients with mild cognitive impairment (MCI) compared to patients without MCI. Insular cortex atrophy also correlated with executive dysfunction in the PD-MCI group (Lee et al. 2013; Mak et al. 2014). Moreover, in Dementia with Lewy bodies (DLB), an entity similar to PD with early onset dementia, the anterior insula was shown to be atrophic in patients during the prodromal phase of the disease (Blanc et al. 2016). Data on the insula’s dense connections, richness of interconnections, central position in networks, and variety of functions indicate that it plays a central role in the brain as a structural hub (van den Heuvel and Sporns 2013). However, the precise involvement of the insular sub-regions in neurodegenerative diseases and their associated functional deficits remain as yet undefined.
VBM, used in the analysis of in vivo structural imaging, has gained much popularity due to its relative user-friendliness and its capacity to reveal changes in brain volumes as indicators of neurodegeneration (Ashburner and Friston 2000). VBM assesses changes in whole brain volumes using a voxel-by-voxel comparison of grey or white matter between groups through parametric tests and a user set p value (Whitwell 2009). It is often difficult, however, to compare across individual studies using VBM, due to limited sample sizes, the use of different corrections, registration and pre-processing tools (Whitwell 2009). This could in turn lead to false positive and negative results as well as inconsistent findings, hence limiting the reliability of individual studies. Therefore, in this study, we used a meta-analytic approach across multiple studies to quantitatively identify the insular sub-regions that consistently showed significant differences across disease groups.
Considering the heterogeneity of the insular cortex and its potential contribution to a myriad of functions affected in neurodegenerative diseases, we aimed to systematically collect and quantitatively analyze data on insular cortex sub-regional atrophy in neurodegenerative disorders. We hypothesized that selective atrophy of the anterior insular cortex would be associated with cognitive and neuropsychiatric deficits in neurodegenerative diseases. To address this issue, we studied insular cortex sub-regional atrophy and its contribution to cognitive and neuropsychiatric deficits across FTD, AD, PD and DLB using Anatomic Likelihood Estimation (ALE) as a meta-analytic quantitative approach.
Methods
Systematic search
This systematic review is reported according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) (Moher et al. 2010). Search syntaxes were tailored to retrieve VBM studies assessing the role of the insular cortex in AD, FTD, PD, and DLB using two bibliographic databases: PubMed and Embase. Search syntaxes included a combination of the following: (“insula” OR “von Economo neurons”) AND (Parkinson’s OR Dementia Lewy body OR frontotemporal OR Alzheimer’s) AND (imaging OR networks OR voxel based morphometry) AND (cognitive impairment OR dementia OR affective OR behavioral OR neuropsychiatry) as well as their appropriate Medical Subject Headings (MeSH). The search was run in January 2017. Articles were then screened using a predefined eligibility criteria: 1) English language 2) includes human subjects only 3) Peer-reviewed studies 4) Primary studies only 5) Studies on any of the four diseases mentioned including the FTD disorders such as semantic dementia, behavioral variant FTD (bvFTD), and progressive aphasias. Studies were included regardless of the presence of a healthy control group. For the meta-analysis, studies were included if they reported the insular coordinates in Talairach or MNI space. The stereotactic coordinates of the insular cortex were included from whole-brain analyses. Authors were contacted, when necessary, to retrieve the exact insular coordinates or in case of missing information. When coordinates were not retrieved, studies were excluded from the meta-analysis. References of the included publications were screened for additional relevant studies.
Following a similar meta-analytic approach, studies were then categorized into 6 main functional domains: cognition, speech, emotion, perception, behavior, and affective-cognition. Studies that assessed an emotion or complex emotional states such as apathy were categorized in the emotional domain. Similarly, studies that assessed a cognitive function were added to the cognitive domain. The affective-cognition category included functions with both emotional and cognitive aspects such as empathy, the recognition of an emotion or enhancement of cognition through emotion. Categories were defined based on the type of functions retrieved as performed in a similar study (Criaud et al. 2016).
Meta-analysis included assessing 1) insular cortex atrophy per disease group and across all groups combined: FTD, AD, PD, and DLB, and 2) assessment of functional domains and their relationship with atrophy in patients compared to controls. A whole-group analysis was performed to define convergence clusters of atrophy in the insular cortex across all diseases together. Each disease group was then assessed separately, while PD and DLB studies were combined (PD/DLB group) due to the small number of DLB studies as well as similarities between the two diseases. To compare insular contribution to each disease, we performed contrast studies: AD versus FTD, AD versus PD/DLB, and FTD versus PD/DLB. Subsequently, conjunction maps were plotted to identify overlapping insular sub-regions of atrophy across the three defined disease groups. Meanwhile, we assessed the cognitive and psychiatric correlates, categorized by functional domain, of atrophy in the insular cortex across the above-mentioned neurodegenerative diseases. Only studies that assessed a correlation between atrophy and a functional domain, regardless of the presence of control groups, were included in the meta-analysis. Peak insular coordinates derived from correlation between VBM atrophy and function were included. An anatomical map with central sulcus as a landmark was used to separate between the anterior and posterior insular divisions. The anterior insula was divided into ventral and dorsal sub-regions based on the macro- and microscopic landmarks as previously studied (Naidich et al. 2004; Morel et al. 2013) (Fig. 1).
Insular Cortex Sub-regions in MRI. The central sulcus generally divides the insular cortex into anterior and posterior subdivisions. The anterior insular cortex is further divided into ventral (red) and dorsal sub-regions (green) and the dorsal insula can be divided into an anterior and mid-region. The left figure shows the sub-regions of the insular cortex based on the Hammers_mith probabilistic atlas (Faillenot et al., 2017). Surface rendering of the insular sub-regions in 3D is shown on the right. A: anterior, dAI: dorsal anterior insula, I: inferior, P: posterior, PI: posterior insula, S: superior, vAI: ventral anterior insula
Statistical analysis
Analysis of the VBM data was performed using an Anatomic Estimation Likelihood (ALE) approach, using GingerALE 2.3.5 (http://brainmap.org/ale/). This meta-analytic technique uses the coordinates of studies in which significant differences were observed and displays them as 3D Gaussian probability distributions (Laird et al. 2005). The MNI coordinates of insular foci were extracted from each study and converted to Talairach using Lancaster transform applied in GingerALE (Eickhoff et al. 2009).
Statistical significance in single studies was determined using an uncorrected p < 0.001 and cluster size 200mm3. Other available approaches for the computation of convergence of anatomic probabilities existing above chance include False discovery rate correction (FDR), Family wise error correction (FWE), and cluster level analysis, which are commonly used at a p value <0.05. The latter approach, although more sensitive than FDR and FWE and protects from false positives, may not be suitable for VBM studies as previously mentioned (Ashburner and Friston 2000; Eickhoff et al. 2012). Moreover, as the FDR and FWE approaches are more conservative, they would yield very restricted results (Eickhoff et al. 2012). An uncorrected p was used in this analysis as a quick and deterministic analytical method since only insular cortex rather than whole-brain coordinates were used. Contrast analyses used the same parameters as well as permutation testing with 10,000 iterations. Clusters of atrophy in the insula from different studies were then mapped on a Colin-27 template in Talairach space using Mango software for image processing (www.ric.uthscsa.edu/mango).
Results
Systematic search
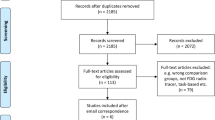
The systematic search through Embase and Pubmed yielded 519 studies. After title and abstract screening and removal of duplicates, 159 studies qualified for full text examination. A total of 46 original articles fulfilled the inclusion criteria and were included in this review. For the meta-analysis, the stereotactic insular coordinates of atrophy were retrieved from a total of 41 studies fulfilling the eligibility criteria. The total number of subjects in the included studies is 2261. There are 25 studies on FTD, 21 on AD (including 11 studies comparing FTD and AD), 11 studies on PD, and 2 on DLB (both comparing DLB and AD). Figure 2 shows a detailed flow diagram of the study selection process.
PRISMA flow Diagram of study selection. Diagram shows the search results, removal of duplicates, and final articles included after title and abstract screening and full text examination. A total of 46 studies fitting the inclusion criteria were added. The functional categories assessed in meta-analysis include speech, cognition, emotion, perception, affective-cognition, and behavior
The cognitive domain included studies on cognitive impairment assessed by global cognitive tests (n = 7), theory of mind, assessing the ability to place oneself in other’s minds (n = 2), and self-awareness (n = 1). The emotion domain included studies on apathy (n = 4), happiness (n = 1), music aversion (n = 1), fear conditioning and emotional blunting (n = 1). The affective-cognitive domain contained studies on recognition of emotions and emotional enhancement of cognition (n = 6). The perception domain included delusions (n = 2) and hallucinations (n = 3). Behavior contained studies on aberrant eating (n = 4), disgust behavior (n = 1), pathological gambling (n = 1), auditory hedonic behavior (n = 1) and prosocial behavior (n = 1). The domain speech included aphasia, verbal agility, and speech fluency (n = 3) (Supplementary Table 1). There were 2 studies on pain, temperature, and blood pressure. Two further studies reported a relationship between the insula and activities of daily living. The latter studies were not included in the meta-analysis due to the heterogeneity of functions. In the meta-analysis, peak insular coordinates were pooled and analyzed if they corresponded to any of the following domains: cognition, emotion, perception, speech, affective-cognition, and behavior (Table 1).
Literature review
Insular atrophy associated deficits in FTD
Studies on FTD showed a positive correlation between left insular atrophy and speech deficits such as verbal agility and verbal fluency, defined by words per minute (Ash et al. 2009; Mandelli et al. 2016). Insular atrophy was also found in FTD patients suffering from aphasia when compared with AD patients (Hu et al. 2010). Other studies found a positive correlation between insular atrophy and fear conditioning deficits, happiness, empathy deficits, and deficits in the recognition of emotion or enhancement of memory through emotion (Hoefer et al. 2008; Omar et al. 2011; Hsieh et al. 2012; Couto et al. 2013; Kumfor et al. 2013, 2014; Cerami et al. 2014; Sturm et al. 2015; Dermody et al. 2016). Kipps et al. reported that bvFTD patients with significant insular atrophy performed worse in recognizing negative emotions compared to positive ones, when compared with AD, controls, and bvFTD without significant insular atrophy (Kipps et al. 2009). Insular atrophy also positively correlated with behavioral deficits such as disgust, disinhibition, aberrant eating behavior, and compliance to social norms (Woolley et al. 2007; Whitwell et al. 2007; Perry et al. 2014; Woolley et al. 2015; O'Callaghan et al. 2016). One study showed a positive relationship between impaired activities of daily living in FTD with insular atrophy (Amanzio et al. 2016). Fletcher et al. reported a positive association between right middle and posterior insular atrophy and altered pain and temperature responsiveness while Sturm et al. reported higher cardiovascular activity and happiness with left anterior insular atrophy in FTD (Fletcher et al. 2015a; Sturm et al. 2015). Seeley et al. showed that the anterior insula was atrophic in FTD patients with low clinical dementia rating scale (CDR) scores and posterior insular atrophy in patients with higher CDR scores and thus, more cognitive impairment (Seeley et al. 2008). Left anterior insular atrophy also positively correlated with apathy in bvFTD (Eslinger et al. 2012). Rosen et al. showed that right anterior insular atrophy correlated with apathy, disinhibition, eating disorders, and aberrant motor behavior in FTD/semantic dementia (Rosen et al. 2005).
Cognitive and neuropsychiatric deficits in AD and PD
In AD studies, impairment in self-awareness and overestimation of one’s functions positively correlated with right anterior insular atrophy (Shany-Ur et al. 2014). General cognitive performance, assessed by Alzheimer’s disease assessment scale (ADAS), also positively correlated with insular atrophy (Farrow et al. 2007). Moreover, psychosis, including hallucinations and delusions, positively correlated with insular atrophy (Blanc et al. 2014; Ting et al. 2015). One study found a positive correlation between deficits in the recognition of emotions and bilateral insular atrophy (Li et al. 2016). Hu et al. found a positive correlation between agitation and insular atrophy in a cohort of MCI and AD (Hu et al. 2015). Disability in activities of daily living were assessed using Disability assessment for Dementia and showed a positive correlation with insular atrophy (Vasconcelos et al. 2011). In AD, the emotional component of apathy, emotional blunting, rather than the behavioral was associated with left insular atrophy (Stanton et al. 2013). While in PD, apathy correlated with insular atrophy as well as executive dysfunction and cognitive deficits (Reijnders et al. 2010; Alzahrani et al. 2016). Cognitive impairment studies in PD, however, showed variable results. Two studies showed insular atrophy in PD-MCI and PD dementia patient groups; while Lee et al. showed similar results as well as a correlation between insular atrophy and MMSE scores (Song et al. 2011; Lee et al. 2013; Zhang et al. 2015). Similarly, Mak et al. reported insular atrophy in PD and a negative correlation with executive functions (Mak et al. 2014). However, Lee JE et al. showed insular atrophy was present in PD with cognitive impairment but no correlation with executive functions (Lee et al. 2014). Chen et al. found bilateral insular atrophy in PD with normal cognition compared to controls but did not find a correlation with cognitive performance. On the other hand, the authors found a positive correlation between right insular atrophy and UPDRS III scores, indicative of a relationship with motor performance and disease progression (Chen et al. 2016). Gamma et al. reported left insular atrophy in PD patients with visual hallucinations but not cognitive impairment and Shine et al. reported bilateral anterior insular atrophy in correlation with bistable percept paradigm scores for the assessment of visual hallucinations in PD (Gama et al. 2014; Shine et al. 2014). One study assessed pathological gambling in PD but did not find a correlation with insular atrophy (Cerasa et al. 2014). Whereas in DLB, Blanc et al. reported bilateral insular atrophy in patients with MCI in the prodromal phase of the disease, while Heitz et al. found theory of mind deficits associated with attributing mental states to one-self and others, in correlation with insular atrophy in DLB patients (Blanc et al. 2016; Heitz et al. 2016).
Summary of deficits related to insular atrophy in neurodegeneration
Results from VBM studies show that insular cortex atrophy is related to functional deficits in all four diseases. In FTD, insular cortex atrophy was related to speech and language deficits, emotional, affective-cognitive deficits, as well several behavioral deficits. In AD and PD/DLB, insular cortex atrophy was mostly related to psychosis and cognitive impairment. Although the regional involvement of the insula has been variable across studies, the anterior insula has been implicated in multiple deficits.
Meta-analysis
Disease group analysis
Whole-group analysis of atrophy in all disease groups showed a significant convergence cluster of atrophy in the left insula (total: 14136 mm3). Two clusters were present in the right anterior and mid-insula combined (total:11200 mm3). Individual analyses were then performed for each disease group. FTD studies showed a large cluster of atrophy including most of the left insula (10,128 mm3) and several sub-regions in the right insula (total: 6584 mm3). AD studies showed involvement of the bilateral insula with a larger cluster of atrophy in the right insula (2424 mm3). In the PD/DLB group, a significant cluster was found in the right anterior mid-insula (2264 mm3) followed by two smaller clusters in ventral anterior and posterior left insula (total: 2536 mm3) (Fig. 3).
Comparison of insular sub-regional atrophy across diseases
Contrast analyses performed between FTD-AD, FTD-PD/DLB, and AD-PD/DLB did not reveal any clusters of significant difference. There were, however, overlapping clusters of insular atrophy between diseases retrieved from conjunction analyses. Insular regions with overlapping atrophy between AD and FTD showed bilateral anterior and posterior clusters. FTD-PD studies showed an area of atrophy in right mid-insula, corresponding to the posterior short gyrus of the anterior insula and anterior and mid-left insula, both microscopically corresponding to the dorsal dysgranular insula. AD-PD studies showed an overlap in the right mid-insula and two clusters in left anterior and posterior insula (Fig. 4).
Analysis of functional domains related to insular atrophy
Analysis of all six functional domains across all diagnoses showed hemispheric asymmetry with more prominent left insular atrophy. Left insular cortex atrophy was more pronounced in relation to speech, emotion, and affective-cognitive deficits, while the right insula showed greater atrophy in relation to cognitive impairment and perception deficits. Two clusters of atrophy extending through the left dorsal-anterior insular gyrus were found in relation to speech deficits (1288 mm3). In perception, a cluster was found in the right posterior dorsal insula followed by a smaller cluster in the left anterior dorsal insula (528 mm3 and 312 mm3). Affective-cognitive deficits were associated with atrophy in the left dorsal anterior insula (1128 mm3). Meta-analysis of emotional deficits associated with insular atrophy revealed multiple atrophic foci in anterior mid-dorsal and ventral posterior left insula (total: 2400 mm3). Cognitive impairment was associated with bilateral insular atrophy, comprising a larger cluster in the right anterior and mid-dorsal insula (total: 4232 mm3). Analysis of the behavioral functional domain revealed a large cluster in the right posterior insula and smaller clusters in left anterior and posterior ventral insula (1488 mm3 and 760 mm3). Therefore, the anterior dorsal insula was significantly atrophic in all domains except the behavioral domain which showed scattered clusters of atrophy in anterior and posterior insular cortex (Fig. 5).
Insular atrophy and relationship with functional deficits in neurodegeneration. The threshold was set at uncorrected p < 0.001 and cluster size 200 mm3. The main functional domains showing deficits in relation to insular atrophy were speech, perception, emotion, affective-cognitive, cognition, and behavior. The left insula was affected in all domains except cognition where the right insula showed larger clusters in anterior and mid-dorsal insula. Coordinates are in Talairach space. L = left; R = right
Discussion
Knowledge of the sub-regional insular cortex atrophy can provide insight into the selective vulnerability of the insular cortex and its associated clinical deficits in neurodegeneration. Based on the ALE analysis, our results illustrated greater atrophy of the left insular cortex across all diseases combined. Furthermore, the left insular cortex showed larger clusters of atrophy in FTD, compared to PD and AD which in turn showed larger convergence clusters of atrophy in the right insular cortex. Contrasting between all disease groups did not reveal any clusters of atrophy, signifying the lack of disease-specific sub-regional atrophy in the insular cortex. Conjunction analysis, assessing overlapping insular sub-regions of atrophy, showed overlapping clusters in the left anterior insula and right mid-dorsal insula across all diseases. Insular atrophy in FTD was related to emotional, speech, affective-cognitive and behavioral deficits. While atrophy in AD and PD/DLB was mostly associated with perception deficits such as psychosis as well as cognitive impairment. Left insular atrophy was also related to speech, emotional, and affective-cognitive deficits. Atrophy of the right insula, however, was associated with cognitive impairment and perception deficits. Whereas behavioral deficits were related to small clusters in bilateral insula.
Vulnerability of the anterior dorsal insular cortex in neurodegeneration
Analysis has shown involvement of the anterior mid-dorsal insular cortex in the majority of the diseases and functional domains assessed. The anterior dorsal insula has diverse connections to limbic and cortical structures. It is preferentially connected to the orbitofrontal gyrus, olfactory cortex, entorhinal cortex, temporal pole, cingulate gyrus, and parietal cortex (Mesulam and Mufson 1982). Moreover, functional analysis of the insular sub-divisions revealed high functional diversity of this sub-region suggesting it plays an important role in integrating information necessary for cognitive functions. Due to such rich connectivity and diverse functional profile, the dorsal anterior insula could function as a brain hub (Cerliani et al. 2012; Uddin et al. 2014).
Insular cortex atrophy-associated deficits in neurodegeneration
A variety of deficits associated with insular atrophy were found in FTD. These included deficits in understanding emotions and behaviors, speech and language deficits such as aphasia and fluency, emotional deficits such as fear and apathy, and multiple behavioral deficits including aberrant eating and disgusting behavior. Alterations in pain, temperature and physiological reactivity were also associated with insular cortex atrophy in FTD. Whereas cognitive impairment, lack of awareness and psychotic symptoms, such as delusions and visual hallucinations, were related to insular atrophy in AD as well as in PD/DLB. Several studies have also outlined the differential sub-regional involvement of the insula depending on disease stage and severity. Seeley et al. found that the anterior insula was atrophic in FTD patients with low CDR scores while those with high CDR scores and more severe cognitive impairment also had posterior insular atrophy. Similarly, Vasconcelos et al. found right anterior insular atrophy in subjects with mild AD and in correlation with disability assessment for dementia scores as well as MMSE. Although insular atrophy was commonly found in PD with MCI or dementia compared to PD with intact cognition, not all studies found a correlation with cognitive performance. This could result from the use of various cognitive tests that represent global cognitive functions rather than those related to the insular cortex such as salience processing and control of attention based on surrounding stimuli (Uddin 2015). Although it remains unclear how insular atrophy differs in early versus late disease stages, some studies highlight the role of the anterior insula in early disease stages.
Language and speech
Language and speech, verbal agility, verbal fluency, and primary progressive aphasia showed convergence of atrophy in the left anterior mid-dorsal insula in FTD studies. This is in line with other studies showing that the left mid-dorsal insula is activated in response to speech perception tasks (Oh et al. 2014). Speech and language processing are lateralized functions depending more strongly on the left hemisphere and commonly show deficits in FTD (Hickok and Poeppel 2007; Reilly et al. 2010). Likewise, the anterior insula is a region of selective degeneration in FTD (Seeley 2010). It plays a role in the motor control of speech as well as the autonomic elements associated with it such as respiratory control (Ackermann and Riecker 2010). In a study on isolated strokes in the insular cortex, subjects that had left insular lesions exhibited aphasia while lesions in the right hemisphere were related to dysarthria (Baier et al. 2013). Nevertheless, results from various studies, on the precise involvement of the insular cortex in speech production remain inconclusive (Gasquoine 2014). Since speech is controlled bilaterally by the insula (Oh et al. 2014), while this type of aphasia typically involves the left hemisphere, it is possible that by including aphasia and speech in one analysis, our results favor the left hemisphere. Therefore, we re-analyzed the data on speech excluding aphasia but found no difference. Moreover, due to a limited sample size for speech, we analyzed aphasia and fluency together.
Emotional and affective-cognitive deficits in neurodegeneration
Furthermore, emotional deficits including apathy, fear, agitation, and happiness were associated with convergence of atrophy in the left ventral posterior & bilateral mid-dorsal insula. Analysis of apathy only showed similar clusters of atrophy. Apathy is defined by lack of emotions, motivation, and interest and constitutes a common finding in patients with neuropsychiatric disorders. In AD and PD patients, up to 70% and 90% of patients suffer from apathy, respectively (Cummings et al. 2015). Apathy was also found to predict progression from normal cognition to MCI and from MCI to dementia in AD patients (Guercio et al. 2015). Apathy is a complex disorder including an emotional component related to the reward of completing an action as well as a cognitive component for the execution of an action manifesting as lack of goal-directed behavior (Boublay et al. 2016; Kos et al. 2017). The anterior insular cortex also represents a primary structure involved in salience processing which, by detecting salient stimuli and directing attention, provides a motivational context to external stimuli (Menon and Uddin 2010). This could explain the role of the insular cortex in apathy.
Similarly, complex functions related to enhancement of memory through emotions, recognition of emotions, and empathy were particularly affected in FTD. The analysis of this affective-cognitive category revealed a cluster in the left anterior dorsal region. Deficits in recognizing emotions or behaviors occur in several neurodegenerative diseases including FTD, PD, and AD. These functions are important for the development of proper interpersonal skills and constitute multiple underlying functions such as perception and social judgement (Goodkind et al. 2015). Degeneration of the anterior dorsal insula could thus contribute to the processing of functions involving multiple domains due to its role in emotional and cognitive processing.
Insular atrophy behind hallucinations and delusions in AD and PD
Perception deficits including hallucinations and delusions, which both represent psychotic symptoms, were present in AD, PD and DLB patients. Analysis showed a cluster of atrophy in the right dorsal posterior insula and a smaller cluster in the left anterior dorsal insula. Hallucinations are perceptions generated by the mind that exist without the presence of external stimuli, while delusions are abnormal and false beliefs ranging from prosecutory to content specific (Padilla and Mendez 2016). Hallucinations and delusions are common in several neurodegenerative disorders and can have significant burdening effects on patients due to their intrusive nature (Burghaus et al. 2012). The insular cortex functions in integrating internal information and external sensory inputs (Mesulam and Mufson 1982). The anterior part of the insula also plays a role in self-awareness and attention (Craig 2009). Dysfunction of attention networks including the insular cortex could thus contribute to visual hallucinations (Shine et al. 2014). Last, the behavioral deficits, which were most common in FTD, included aberrant eating and disgust behaviors. This domain was associated with atrophy of multiple sub-regions of bilateral insula yet it remains unclear how insular atrophy could contribute to these deficits.
Limitations and future perspectives
In this meta-analysis, we only studied the role of the insular cortex in disease. Since higher functions such as cognition and emotion are the result of an interplay between various regions and networks, focusing only on the insular cortex is a limitation of this study. Nevertheless, studies have identified the role of various brain regions in networks and their subsequent involvement in behavior but information on the contribution of individual brain regions to function has been lacking (Genon et al. 2018). Similarly, the insular cortex has been implicated to play a central role in the salience network and is identified as a hub affected in various diseases and behaviors, yet it has been unclear precisely what contribution it has to the deficits associated with neurodegeneration and whether vulnerability to degeneration is indeed variable across its sub-divisions. Moreover, the focus of this review is only on VBM studies, other neuroimaging modalities were not included. Although VBM is an automated method to assess volume changes across conditions, it is affected by variation in methodologies such as scanner and image quality, pre-processing type, and statistical analysis (Scarpazza et al. 2015). Other limitations are related to technical differences across studies such as smoothing kernel sizes, thresholds, significance levels, and method used for correction of multiple comparison (Whitwell 2009). On the other hand, VBM is an unbiased method used to detect subtle brain changes. It has been useful in identifying regional differences in grey and white matter, common in neurodegenerative diseases, and has shown regional differences in atrophy across diseases as well as relationships with other clinical deficits (Whitwell and Jack Jr 2005; Whitwell and Josephs 2007). ALE is a peak-based method of meta-analysis which relies on pooling peak coordinates rather than using raw maps and thus may yield less accurate results. Furthermore, in this meta-analysis we used an uncorrected p < 0.001 which, despite its conservative value, may have led to false positive results. As previously mentioned, while cluster analysis may have been more sensitive, its use is debatable for VBM studies. Similarly, FDR and FWE approaches are conservative and would have yielded limited results. Since we used a region-of-interest approach by focusing on the insular cortex only, we wanted to be maximally sensitive to small yet meaningful effects. Hence, we opted to use a more liberal methodological approach, uncorrected p, for this meta-analysis. Overall, this study provides a comprehensive summary and quantitative analysis of the relationship between insular cortex sub-regional atrophy across four common neurodegenerative diseases and their corresponding cognitive and neuropsychiatric deficits. Due to the heterogeneity of the insular cortex sub-regions, both structurally and functionally, it is imperative to understand how these regions contribute differentially to neurodegenerative diseases. Even though neuroimaging studies have shed much light onto the role of the insular cortex in disease, several limitations exist. Namkung et al. have recently proposed translational and back-translational approaches to unravel the complex role of the insular cortex. To study causal relationships, Granger causality analysis as well as further computational models and statistical efforts for neuroimage processing along with non-invasive imaging modalities could be used. Furthermore, animal studies could shed some light on the contribution of various neural circuitry elements to behavior. Therefore, to obtain a comprehensive understanding of the complex physiological role of the insular cortex as well as contribution to disease, more research at molecular and cellular levels combined with advanced neuroimaging analyses would be needed (Namkung et al. 2017).
Conclusions
Our meta-analysis showed that atrophy of the insular sub-regions is not disease-specific. Yet, the anterior and middle dorsal insula were atrophic in all included neurodegenerative diseases. The anterior insular cortex, particularly the dorsal insula comprising of a diverse array of limbic and cortical connections, contributed to a broad range of deficits in all neurodegenerative diseases. Our study illustrates the presence of specific patterns of atrophy in the anterior dorsal insula in neurodegeneration which are associated with deficits in speech, emotional, affective-cognitive, perception and cognition. These patterns of insular sub-regional atrophy could aid in understanding clinical heterogeneity in neurodegenerative diseases and provide potentially beneficial information for future biomarker studies.
Abbreviations
- AD:
-
Alzheimer’s disease
- ADAS-TES:
-
Alzheimer’s disease assessment scale- Total error score
- bvFTD:
-
behavioral variant frontotemporal dementia
- CDR:
-
Clinical Dementia Rating
- dAI:
-
Dorsal anterior insula
- DLB:
-
Dementia with Lewy bodies
- FM:
-
family member
- FTD:
-
Frontotemporal dementia
- FWE:
-
Family-wise error
- HC:
-
Healthy controls
- LPA:
-
Logopenic aphasia
- MCI:
-
mild cognitive impairment
- MCI:
-
Mild cognitive impairment
- nfvPPA:
-
non-fluent variant primary progressive aphasia
- PD:
-
Parkinson’s disease
- PDD:
-
Parkinson’s disease dementia
- PI:
-
Posterior insula
- PNFA:
-
Progessive non-fluent aphasia
- PSP:
-
Progressive supranuclear palsy
- rtFTD:
-
right temporal variant FTD
- SD:
-
Semantic dementia
- Soc/Exec:
-
Social/ executive disorder FTD
- SPGI:
-
Superior precentral region of the dorsal anterior insula
- TOM:
-
Theory of mind
- vAI:
-
Ventral anterior insula
References
Ackermann, H., & Riecker, A. (2010). The contribution(s) of the insula to speech production: A review of the clinical and functional imaging literature. Brain Structure & Function, 214, 419–433. https://doi.org/10.1007/s00429-010-0257-x.
Allman, J. M., Tetreault, N. A., Hakeem, A. Y., & Park, S. (2011). The von Economo neurons in apes and humans. American Journal of Human Biology, 23, 5–21. https://doi.org/10.1002/ajhb.21136.
Alzahrani, H., Antonini, A., & Venneri, A. (2016). Apathy in mild Parkinson's disease: Neuropsychological and neuroimaging evidence. Journal of Parkinson's Disease, 6, 821–832. https://doi.org/10.3233/JPD-160809.
Amanzio, M., D'Agata, F., Palermo, S., Rubino, E., Zucca, M., Galati, A., Pinessi, L., Castellano, G., & Rainero, I. (2016). Neural correlates of reduced awareness in instrumental activities of daily living in frontotemporal dementia. Experimental Gerontology, 83, 158–164. https://doi.org/10.1016/j.exger.2016.08.008.
Ash, S., Moore, P., Vesely, L., Gunawardena, D., McMillan, C., Anderson, C., Avants, B., & Grossman, M. (2009). Non-fluent speech in frontotemporal lobar degeneration. Journal of Neurolinguistics, 22, 370–383.
Ashburner, J., & Friston, K. J. (2000). Voxel-based morphometry--the methods. Neuroimage, 11, 805–821. https://doi.org/10.1016/j.jneuroling.2008.12.001.
Augustine, J. (1996). Circuitry and functional aspects of the insular lobe in primates including humans. Brain Research. Brain Research Reviews, 22, 229–244.
Baier, B., Conrad, J., Zu Eulenburg, P., Best, C., Müller-Forell, W., Birklein, F., & Dieterich, M. (2013). Insular strokes cause no vestibular deficits. Stroke, 44, 2604–2606. https://doi.org/10.1161/STROKEAHA.113.001816.
Blanc, F., Noblet, V., Philippi, N., Cretin, B., Foucher, J., Armspach, J. P., Rousseau, F., & Alzheimer's Disease Neuroimaging Initiative. (2014). Right anterior insula Core region of hallucinations in cognitive neurodegenerative diseases. PLoS One, 9, e114774. https://doi.org/10.1371/journal.pone.0114774.
Blanc, F., Colloby, S. J., Cretin, B., de Sousa, P. L., Demuynck, C., O'Brien, J. T., Martin-Hunyadi, C., McKeith, I., Philippi, N., & Taylor, J. P. (2016). Grey matter atrophy in prodromal stage of dementia with Lewy bodies and Alzheimer's disease. Alzheimer's Research & Therapy, 8. https://doi.org/10.1186/s13195-016-0198-6.
Boublay, N., Schott, A. M., & Krolak-Salmon, P. (2016). Neuroimaging correlates of neuropsychiatric symptoms in Alzheimer's disease: A review of 20 years of research. European Journal of Neurology, 23, 1500–1509. https://doi.org/10.1111/ene.13076.
Burghaus, L., Eggers, C., Timmermann, L., Fink, G. R., & Diederich, N. J. (2012). Hallucinations in neurodegenerative diseases. CNS Neuroscience & Therapeutics, 18, 149–159. https://doi.org/10.1111/j.1755-5949.2011.00247.x.
Cerami, C., Dodich, A., Canessa, N., Crespi, C., Marcone, A., Cortese, F., Chierchia, G., Scola, E., Falini, A., & Cappa, S. F. (2014). Neural correlates of empathic impairment in the behavioral variant of frontotemporal dementia. Alzheimer's & Dementia, 10, 827–834. https://doi.org/10.1016/j.jalz.2014.01.005.
Cerasa, A., Salsone, M., Nigro, S., Chiriaco, C., Donzuso, G., Bosco, D., Vasta, R., & Quattrone, A. (2014). Cortical volume and folding abnormalities in Parkinson's disease patients with pathological gambling. Parkinsonism & Related Disorders, 20, 1209–1214. https://doi.org/10.1016/j.parkreldis.2014.09.001.
Cerliani, L., Thomas, R. M., Jbabdi, S., Siero, J. C., Nanetti, L., Crippa, A., Gazzola, V., D'Arceuil, H., & Keysers, C. (2012). Probabilistic tractography recovers a rostrocaudal trajectory of connectivity variability in the human insular cortex. Human Brain Mapping, 33, 2005–2034. https://doi.org/10.1002/hbm.21338.
Chen, F. X., Kang, D. Z., Chen, F. Y., Liu, Y., Wu, G., Li, X., Yu, L. H., Lin, Y. X., & Lin, Z. Y. (2016). Gray matter atrophy associated with mild cognitive impairment in Parkinson’s disease. Neuroscience Letters, 617, 160–165. https://doi.org/10.1016/j.neulet.2015.
Christopher, L., Koshimori, Y., Lang, A. E., Criaud, M., & Strafella, A. P. (2014). Uncovering the role of the insula in non-motor symptoms of Parkinson's disease. Brain, 137, 2143–2154. https://doi.org/10.1093/brain/awu084.
Couto, B., Manes, F., Montañés, P., Matallana, D., Reyes, P., Velasquez, M., Yoris, A., Baez, S., & Ibáñez, A. (2013). Structural neuroimaging of social cognition in progressive non-fluent aphasia and behavioral variant of frontotemporal dementia. Frontiers in Human Neuroscience, 7. https://doi.org/10.3389/fnhum.2013.00467.
Craig, A. D. (2009). How do you feel--now? The anterior insula and human awareness. Nature Reviews. Neuroscience, 10, 59–70. https://doi.org/10.1038/nrn2555.
Criaud, M., Christopher, L., Boulinguez, P., Ballanger, B., Lang, A. E., Cho, S. S., & Houle, S. A. P. (2016). Contribution of insula in Parkinson's disease: A quantitative meta-analysis study. Human Brain Mapping, 37, 1375–1392. https://doi.org/10.1002/hbm.23109.
Crossley, N. A., Mechelli, A., Scott, J., Carletti, F., Fox, P. T., McGuire, P., & Bullmore, E. T. (2014). The hubs of the human connectome are generally implicated in the anatomy of brain disorders. Brain, 137, 2382–2395. https://doi.org/10.1093/brain/awu132.
Cummings, J., Friedman, J. H., Garibaldi, G., Jones, M., Macfadden, W., Marsh, L., & Robert, P. H. (2015). Apathy in neurodegenerative diseases: Recommendations on the Design of Clinical Trials. Journal of Geriatric Psychiatry and Neurology, 28, 159–173. https://doi.org/10.1177/0891988715573534.
Dermody, N., Wong, S., Ahmed, R., Piguet, O., Hodges, J. R., & Irish, M. (2016). Uncovering the neural bases of cognitive and affective empathy deficits in Alzheimer's disease and the behavioral-variant of frontotemporal dementia. Journal of Alzheimer's Disease, 53, 801–816. https://doi.org/10.3233/JAD-160175.
Eickhoff, S. B., Laird, A. R., Grefkes, C., Wang, L. E., Zilles, K., & Fox, P. T. (2009). Coordinate-based activation likelihood estimation meta-analysis of neuroimaging data: A random-effects approach based on empirical estimates of spatial uncertainty. Human Brain Mapping, 30, 2907–2926. https://doi.org/10.1002/hbm.20718.
Eickhoff, S. B., Bzdok, D., Laird, A. R., Kurth, F., & Fox, P. T. (2012). Activation likelihood estimation meta-analysis revisited. Neuroimage, 59(3), 2349–2361. https://doi.org/10.1016/j.neuroimage.2011.09.017.
Eslinger, P. J., Moore, P., Antani, S., Anderson, C., & Grossman, M. (2012). Apathy in frontotemporal dementia: Behavioral and neuroimaging correlates. Behavioural Neurology, 25, 127–136. https://doi.org/10.3233/BEN-2011-0351.
Evrard, H. C., Forro, T., & Logothetis, N. K. (2012). Von Economo neurons in the anterior insula of the macaque monkey. Neuron, 74, 482–489. https://doi.org/10.1016/j.neuron.2012.03.003.
Faillenot, I., Heckemann, R. A., Frot, M., & Hammers, A. (2017). Macroanatomy and 3D probabilistic atlas of the human insula. Neuroimage., 150, 88–98. https://doi.org/10.1016/j.neuroimage.2017.01.073.
Farrow, T. F., Thiyagesh, S. N., Wilkinson, I. D., Parks, R. W., Ingram, L., & Woodruff, P. W. (2007). Fronto-temporal-lobe atrophy in early-stage Alzheimer's disease identified using an improved detection methodology. Psychiatric Research, 155, 11–19. https://doi.org/10.1016/j.pscychresns.2006.12.013.
Fletcher, P. D., Downey, L. E., Golden, H. L., Clark, C. N., Slattery, C. F., Paterson, R. W., Rohrer, J. D., Schott, J. M., Rossor, M. N., & Warren, J. D. (2015a). Pain and temperature processing in dementia: A clinical and neuroanatomical analysis. Brain, 138, 3360–3372. https://doi.org/10.1093/brain/awv276.
Fletcher, P. D., Downey, L. E., Golden, H. L., Clark, C. N., Slattery, C. F., Paterson, R. W., Schott, J. M., Rohrer, J. D., Rossor, M. N., & Warren, J. D. (2015b). Auditory hedonic phenotypes in dementia: A behavioural and neuroanatomical analysis. Cortex, 67, 95–105. https://doi.org/10.1016/j.cortex.2015.03.021.
Flynn, F. G. (1999). Anatomy of the insula functional and clinical correlates. Aphasiology, 13, 55–78.
Gama, R. L., Bruin, V. M., Távora, D. G., Duran, F. L., Bittencourt, L., & Tufik, S. (2014). Structural brain abnormalities in patients with Parkinson's disease with visual hallucinations: A comparative voxel-based analysis. Brain and Cognition, 87, 97–103. https://doi.org/10.1016/j.bandc.2014.03.011.
Gasquoine, P. G. (2014). Contributions of the insula to cognition and emotion. Neuropsychology Review, 24, 77–87. https://doi.org/10.1007/s11065-014-9246-9.
Genon, S., Reid, A., Langner, R., Amunts, K., & Eickhoff, S. B. (2018). How to characterize the function of a brain region. Trends in Cognitive Sciences, 22(4), 350–364. https://doi.org/10.1016/j.tics.2018.01.010.
Goodkind, M. S., Sturm, V. E., Ascher, E. A., Shdo, S. M., Miller, B. L., Rankin, K. P., & Levenson, R. W. (2015). Emotion recognition in frontotemporal dementia and Alzheimer's disease: A new film-based assessment. Emotion, 15, 416–427. https://doi.org/10.1037/a0039261.
Guercio, B. J., Donovan, N. J., Munro, C. E., Aghjayan, S. L., Wigman, S. E., Locascio, J. J., Amariglio, R. E., Rentz, D. M., Johnson, K. A., Sperling, R. A., & Marshall, G. A. (2015). The apathy evaluation scale: A comparison of subject, informant, and clinician report in cognitively Normal elderly and mild cognitive impairment. Journal of Alzheimer's Disease, 47, 421–432. https://doi.org/10.3233/JAD-150146.
Heitz, C., Noblet, V., Phillipps, C., Cretin, B., Vogt, N., Philippi, N., Kemp, J., de Petigny, X., Bilger, M., Demuynck, C., Martin-Hunyadi, C., Armspach, J. P., & Blanc, F. (2016). Cognitive and affective theory of mind in dementia with Lewy bodies and Alzheimer's disease. Alzheimer's Research & Therapy, 8, 10. https://doi.org/10.1186/s13195-016-0179-9.
Hickok, G., & Poeppel, D. (2007). The cortical organization of speech processing. Nature Reviews Neuroscience, 8, 393–402. https://doi.org/10.1038/nrn2113.
Hoefer, M., Allison, S. C., Schauer, G. F., Neuhaus, J. M., Hall, J., Dang, J. N., Weiner, M. W., Miller, B. L., & Rosen, H. J. (2008). Fear conditioning in frontotemporal lobar degeneration and Alzheimer's disease. Brain, 131, 1646–1657. https://doi.org/10.1093/brain/awn082.
Hsieh, S., Hornberger, M., Piguet, O., & Hodges, J. R. (2012). Brain correlates of musical and facial emotion recognition: Evidence from the dementias. Neuropsychologia, 50, 1814–1822. https://doi.org/10.1016/j.neuropsychologia.2012.04.006.
Hu, W. T., McMillan, C., Libon, D., Leight, S., Forman, M., Lee, V. M., Trojanowski, J. Q., & Grossman, M. (2010). Multimodal predictors for Alzheimer disease in nonfluent primary progressive aphasia. Neurology, 75, 595–602. https://doi.org/10.1212/WNL.0b013e3181ed9c52.
Hu, X., Meiberth, D., Newport, B., & Jessen, F. (2015). Anatomical correlates of the neuropsychiatric symptoms in Alzheimer's disease. Current Alzheimer Research, 12, 266–277.
Kipps, C. M., Nestor, P. J., Acosta-Cabronero, J., Arnold, R., & Hodges, J. R. (2009). Understanding social dysfunction in the behavioural variant of frontotemporal dementia: The role of emotion and sarcasm processing. Brain, 132, 592–603. https://doi.org/10.1093/brain/awn314.
Kos, C., Klaasen, N. G., Marsman, J. C., Opmeer, E. M., Knegtering, H., Aleman, A., & van Tol, M. J. (2017). Neural basis of self-initiative in relation to apathy in a student sample. Scientific Reports, 7, 3264. https://doi.org/10.1038/s41598-017-03564-5.
Kumfor, F., Irish, M., Hodges, J. R., & Piguet, O. (2013). Discrete neural correlates for the recognition of negative emotions: Insights from frontotemporal dementia. PLoS One, 8, e67457. https://doi.org/10.1371/journal.pone.0067457.
Kumfor, F., Irish, M., Hodges, J. R., & Piguet, O. (2014). Frontal and temporal lobe contributions to emotional enhancement of memory in behavioral-variant frontotemporal dementia and Alzheimer's disease. Frontiers in Behavioral Neuroscience, 8. https://doi.org/10.3389/fnbeh.2014.00225.
Laird, A. R., Fox, P. M., Price, C. J., Glahn, D. C., Uecker, A. M., Lancaster, J. L., Turkeltaub, P. E., Kochunov, P., & Fox, P. T. (2005). ALE meta-analysis: Controlling the false discovery rate and performing statistical contrasts. Human Brain Mapping, 25(1), 155–164. https://doi.org/10.1002/hbm.20136.
Lee, S. H., Kim, S. S., Tae, W. S., Lee, S. Y., Lee, K. U., & Jhoo, J. (2013). Brain volumetry in Parkinson's disease with and without dementia: Where are the differences? Acta Radiologica, 54, 581–586. https://doi.org/10.1177/0284185113476029.
Lee, J. E., Cho, K. H., Song, S. K., Kim, H. J., Lee, H. S., Sohn, Y. H., & Lee, P. H. (2014). Exploratory analysis of neuropsychological and neuroanatomical correlates of progressive mild cognitive impairment in Parkinson's disease. Journal of Neurology, Neurosurgery, and Psychiatry, 85, 7–16. https://doi.org/10.1136/jnnp-2013-305062.
Li, X., Wang, H., Tian, Y., Zhou, S., Li, X., Wang, K., & Yu, Y. (2016). Impaired white matter connections of the limbic system networks associated with impaired emotional memory in Alzheimer's disease. Frontiers in Aging Neuroscience, 8. https://doi.org/10.3389/fnagi.2016.00250.
Mak, E., Zhou, J., Tan, L. C., Au, W. L., Sitoh, Y. Y., & Kandiah, N. (2014). Cognitive deficits in mild Parkinson's disease are associated with distinct areas of grey matter atrophy. Journal of Neurology, Neurosurgery, and Psychiatry, 85, 576–580. https://doi.org/10.1136/jnnp-2013-305805.
Mandelli, M. L., Vitali, P., Santos, M., Henry, M., Gola, K., Rosenberg, L., Dronkers, N., Miller, B., Seeley, W. W., & Gorno-Tempini, M. L. (2016). Two insular regions are differentially involved in behavioral variant FTD and nonfluent/agrammatic variant PPA. Cortex, 74, 149–157. https://doi.org/10.1016/j.cortex.2015.10.012.
Menon, V., & Uddin, L. Q. (2010). Saliency, switching, attention and control: A network model of insula function. Brain Structure and Function, 214, 655–667. https://doi.org/10.1007/s00429-010-0262-0.
Mesulam, M. M., & Mufson, E. J. (1982). Insula of the old world monkey. III: Efferent cortical output and comments on function. The Journal of Comparative Neurology, 212, 38–52. https://doi.org/10.1002/cne.902120104.
Meyer, S., Mueller, K., Stuke, K., Bisenius, S., Diehl-Schmid, J., Jessen, F., Kassubek, J., Kornhuber, J., Ludolph, A. C., Prudlo, J., Schneider, A., Schuemberg, K., Yakushev, I., Otto, M., & Schroeter, M. L. (2017). Predicting behavioral variant frontotemporal dementia with pattern classification in multi-center structural MRI data. Neurologic Clinics, 14, 656–662. https://doi.org/10.1016/j.nicl.2017.02.001.
Moher, D., Liberati, A., Tetzlaff, J., Altman, D. G., & PRISMA Group (2010). Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. International Journal of Surgery, 8, 336–341. https://doi.org/10.1016/j.ijsu.2010.02.007.
Morel, A., Gallay, M. N., Baechler, A., Wyss, M., & Gallay, D. S. (2013). The human insula: Architectonic organization and postmortem MRI registration. Neuroscience, 236, 117–135. https://doi.org/10.1016/j.neuroscience.2012.12.076.
Naidich, T. P., Kang, E., Fatterpekar, G. M., Delman, B. N., Gultekin, S. H., Wolfe, D., Ortiz, O., Yousry, I., Weismann, M., & Yousry, T. A. (2004). The insula: Anatomic study and MR imaging display at 1.5 T. AJNR. American Journal of Neuroradiology, 25, 222–232.
Nakaaki, S., Sato, J., Torii, K., Oka, M., Negi, A., Nakamae, T., Narumoto, J., Miyata, J., Furukawa, T. A., & Minura, M. (2013). Neuroanatomical abnormalities before onset of delusions in patients with Alzheimer's disease: A voxel-based morphometry study. Neuropsychiatric Disease and Treatment, 9, 1–8. https://doi.org/10.2147/NDT.S38939.
Namkung, H., Kim, S. H., & Sawa, A. (2017). The insula: An underestimated brain area in clinical neuroscience, psychiatry, and neurology. Trends in Neurosciences, 40, 200–207. https://doi.org/10.1016/j.tins.2017.02.002.
O'Callaghan, C., Bertoux, M., Irish, M., Shine, J. M., Wong, S., Spiliopoulos, L., Hodges, J. R., & Hornberger, M. (2016). Fair play: social norm compliance failures in behavioural variant frontotemporal dementia. Brain, 139, 204–216. https://doi.org/10.1093/brain/awv315.
Oh, A., Duerden, E. G., & Pang, E. W. (2014). The role of the insula in speech and language processing. Brain and Language, 135, 96–103. https://doi.org/10.1016/j.bandl.2014.06.003.
Omar, R., Henley, S. M., Bartlett, J. W., Hailstone, J. C., Gordon, E., Sauter, D. A., Frost, C., Scott, S. K., & Warren, J. D. (2011). The structural neuroanatomy of music emotion recognition: Evidence from frontotemporal lobar degeneration. Neuroimage, 56, 1814–1821. https://doi.org/10.1016/j.neuroimage.2011.03.002.
Padilla C. R., & Mendez M. F. (2016). Neuropsychiatric features across neurodegenerative diseases. In J. L. Pillai (Ed.), Neurodegenerative diseases: Unifying principles (pp. 85–97). Oxford: Oxford University Press.
Perry, D. C., Sturm, V. E., Seeley, W. W., Miller, B. L., Kramer, J. H., & Rosen, H. J. (2014). Anatomical correlates of reward-seeking behaviours in behavioural variant frontotemporal dementia. Brain, 137, 1621–1626. https://doi.org/10.1093/brain/awu075.
Reijnders, J. S., Scholtissen, B., Weber, W. E., Aalten, P., Verhey, F. R., & Leentjens, A. F. (2010). Neuroanatomical correlates of apathy in Parkinson's disease: A magnetic resonance imaging study using voxel-based morphometry. Movement Disorders, 25, 2318–2325. https://doi.org/10.1002/mds.23268.
Reilly, J., Rodriguez, A. D., Lamy, M., & Neils-Strunjas, J. (2010). Cognition, language, and clinical pathological features of non-Alzheimer's dementias: An overview. Journal of Communication Disorders, 43, 438–452. https://doi.org/10.1016/j.jcomdis.2010.04.011.
Rosen, H. J., Allison, S. C., Schauer, G. F., Gorno-Tempini, M. L., Weiner, M. W., & Miller, B. L. (2005). Neuroanatomical correlates of behavioural disorders in dementia. Brain, 128, 2612–2625. https://doi.org/10.1093/brain/awh628.
Rosenberg, P. B., Nowrangi, M. A., & Lyketsos, C. G. (2015). Neuropsychiatric symptoms in Alzheimer's disease: What might be associated brain circuits? Molecular Aspects of Medicine, 43-44, 25–37. https://doi.org/10.1016/j.mam.2015.05.005.
Scarpazza, C., Tognin, S., Frisciata, S., Sartori, G., & Mechelli, A. (2015). False positive rates in voxel-based morphometry studies of the human brain: Should we be worried? Neuroscience and Biobehavioral Reviews, 52, 49–55. https://doi.org/10.1016/j.neubiorev.2015.02.008.
Seeley, W. W. (2010). Anterior insula degeneration in frontotemporal dementia. Brain Structure & Function, 214, 465–475. https://doi.org/10.1007/s00429-010-0263-z.
Seeley, W. W., Crawford, R., Rascovsky, K., Kramer, J. H., Weiner, M., Miller, B. L., & Gorno-Tempini, M. L. (2008). Frontal Paralimbic network atrophy in very mild behavioral variant frontotemporal dementia. Archives of Neurology, 65, 249–255. https://doi.org/10.1001/archneurol.2007.38.
Shany-Ur, T., Lin, N., Rosen, H. J., Sollberger, M., Miller, B. L., & Rankin, K. P. (2014). Self-awareness in neurodegenerative disease relies on neural structures mediating reward-driven attention. Brain., 137, 2368–2381. https://doi.org/10.1093/brain/awu161.
Shine, J. M., Halliday, G. M., Gilat, M., Matar, E., Bolitho, S. J., Carlos, M., Naismith, S. L., & Lewis, S. J. (2014). The role of dysfunctional attentional control networks in visual misperceptions in Parkinson's disease. Human Brain Mapping, 35, 2206–2219. https://doi.org/10.1002/hbm.22321.
Song, S. K., Lee, J. E., Park, H. J., Sohn, Y. H., Lee, J. D., & Lee, P. H. (2011). The pattern of cortical atrophy in patients with Parkinson's disease according to cognitive status. Movement Disorders, 26, 289–296. https://doi.org/10.1002/mds.23477.
Stanton, B. R., Leigh, P. N., Howard, R. J., Barker, G. J., & Brown, R. G. (2013). Behavioural and emotional symptoms of apathy are associated with distinct patterns of brain atrophy in neurodegenerative disorders. Journal of Neurology, 260, 2481–2490. https://doi.org/10.1007/s00415-013-6989-9.
Sturm, V. E., Yokoyama, J. S., Eckart, J. A., Zakrzewski, J., Rosen, H. J., Miller, B. L., Seeley, W. W., & Levenson, R. W. (2015). Damage to left frontal regulatory circuits produces greater positive emotional reactivity in frontotemporal dementia. Cortex, 64, 55–67. https://doi.org/10.1016/j.cortex.2014.10.002.
Ting, W. K., Fischer, C. E., Millikin, C. P., Ismail, Z., Chow, T. W., & Schweizer, T. A. (2015). Grey matter atrophy in mild cognitive impairment / early Alzheimer disease associated with delusions: A voxel-based morphometry study. Current Alzheimer Research, 12, 165–172.
Uddin, L. Q. (2015). Salience processing and insular cortical function and dysfunction. Nature Reviews. Neuroscience, 16, 55–61. https://doi.org/10.1038/nrn3857.
Uddin, L. Q., Kinnison, J., Pessoa, L., & Anderson, M. L. (2014). Beyond the tripartite cognition-emotion-interoception model of the human insular cortex. Journal of Cognitive Neuroscience, 26(1), 16–27. https://doi.org/10.1162/jocn_a_00462.
Uddin, L. Q., Nomi, J. S., Hébert-Seropian, B., Ghaziri, J., & Boucher, O. (2017). Structure and function of the human insula. Journal of Clinical Neurophysiology, 34, 300–306. https://doi.org/10.1097/WNP.0000000000000377.
van den Heuvel, M. P., & Sporns, O. (2013). Network hubs in the human brain. Trends in Cognitive Sciences, 17, 683–696. https://doi.org/10.1016/j.tics.2013.09.012.
Vasconcelos, L. de G., Jackowski, A. P., Oliveira, M. O., Flor, Y. M., Bueno, O. F., & Brucki, S. M. (2011). Voxel-based morphometry findings in Alzheimer’s disease: neuropsychiatric symptoms and disability correlations - preliminary results. Clinics (Sao Paulo), 66, 1045–1050. https://doi.org/10.1590/S1807-59322011000600021.
Whitwell, J. L. (2009). Voxel-based morphometry: An automated technique for assessing structural changes in the brain. The Journal of Neuroscience, 29, 9661–9664. https://doi.org/10.1523/JNEUROSCI.2160-09.2009.
Whitwell, J. L., & Jack, C. R., Jr. (2005). Comparisons between Alzheimer disease, frontotemporal lobar degeneration, and normal aging with brain mapping. Topics in Magnetic Resonance Imaging, 16, 409–425. https://doi.org/10.1097/01.rmr.0000245457.98029.e1.
Whitwell, J. L., & Josephs, K. A. (2007). Voxel-based morphometry and its application to movement disorders. Parkinsonism & Related Disorders, 13, S406–S416. https://doi.org/10.1016/S1353-8020(08)70039-7.
Whitwell, J. L., Sampson, E. L., Loy, C. T., Warren, J. E., Rossor, M. N., Fox, N. C., & Warren, J. D. (2007). VBM signatures of abnormal eating behaviours in frontotemporal lobar degeneration. Neuroimage, 35, 207–213. https://doi.org/10.1016/j.neuroimage.2006.12.006.
Woolley, J. D., Gorno-Tempini, M. L., Seeley, W. W., Rankin, K., Lee, S. S., Matthews, B. R., & Miller, B. L. (2007). Binge eating is associated with right orbitofrontal-insular-striatal atrophy in frontotemporal dementia. Neurology, 69, 1424–1433. https://doi.org/10.1212/01.wnl.0000277461.06713.23.
Woolley, J. D., Strobl, E. V., Sturm, V. E., Shany-Ur, T., Poorzand, P., Grossman, S., Nguyen, L., Eckart, J. A., Levenson, R. W., Seeley, W. W., Miller, B. L., & Rankin, K. P. (2015). Impaired recognition and regulation of disgust is associated with distinct but partially overlapping patterns of decreased gray matter volume in the Ventroanterior insula. Biological Psychiatry, 78, 505–514. https://doi.org/10.1016/j.biopsych.2014.12.031.
Zhang, J., Zhang, Y. T., Hu, W. D., Li, L., Liu, G. Y., & Bai, Y. P. (2015). Gray matter atrophy in patients with Parkinson's disease and those with mild cognitive impairment: A voxel-based morphometry study. International Journal of Clinical and Experimental Medicine, 8, 15383–15392.
Funding
This study was funded by the Stichting ParkinsonFonds, the Netherlands (https://www.parkinsonfonds.nl/).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare having no conflict of interest.
Ethical approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
ESM 1
(DOCX 15 kb)
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Fathy, Y.Y., Hoogers, S.E., Berendse, H.W. et al. Differential insular cortex sub-regional atrophy in neurodegenerative diseases: a systematic review and meta-analysis. Brain Imaging and Behavior 14, 2799–2816 (2020). https://doi.org/10.1007/s11682-019-00099-3
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11682-019-00099-3