Abstract
Background
Supracondylar fractures of the humerus are the most common type of elbow fractures in childhood. Due to the potential risk of severe complications, trauma surgeons should address them with caution. Avascular necrosis of the trochlea presents a rare but oftentimes disabling complication and should not be underestimated. The aim of the present study was to identify possible predictors of avascular necrosis of the trochlea following pediatric supracondylar humerus fractures.
Methods
We reviewed the available body of literature reporting clinical outcomes, complications, and possible predictors of avascular necrosis of the trochlea after supracondylar humerus fractures in childhood. Data on patient age, sex, the affected side, fracture classification, treatment, the number of K‑wires, time to surgery, complications, and the time from injury to diagnosis of avascular necrosis were obtained. This study was performed according to the PRISMA guidelines.
Results
Eight clinical studies were included, comprising 30 patients with avascular necrosis after supracondylar fractures in childhood. The mean age at the time of injury was 5 years (min. 2; max. 10; SD: 2.8 years). In all, 18 patients (60.0%) were male, 11 (36.7%) were female, and one was unknown (3.3%). Five patients (16.7%) had a Gartland type I, three (10.0%) a type II, and 22 (73.3%) a type III fracture of the distal humerus. Six patients (20.0%) were treated conservatively, whereas 24 patients (80.0%) underwent operative treatment. The mean time from injury to diagnosis of avascular necrosis was 33 months (min. 4; max. 84; SD: 24.5 months).
Conclusion
The available literature on avascular necrosis of the trochlea following pediatric supracondylar humerus fractures is limited. While it can occur in any supracondylar fracture, fracture displacement may be considered a risk factor.
Zusammenfassung
Hintergrund
Suprakondyläre Humerusfrakturen zählen zu den häufigsten Verletzungen des kindlichen Ellenbogens. Aufgrund der potenziell hohen Komplikations- sowie Revisionsrate zählt diese Verletzung auch zu den „Kadiläsionen“. Neben den häufigeren Komplikationen wie den neurovaskulären Begleitverletzungen ist die avaskuläre Nekrose eine Seltenheit, die trotz alldem nicht unterschätzt werden sollte. Das Ziel dieser systematischen Literaturrecherche ist es, mögliche Risikofaktoren herauszuarbeiten.
Methoden und Materialien
Die systematische Literaturrecherche orientiert sich an den PRISMA-Richtlinien (Preferred Reporting Items for Systematic Reviews and Meta-Analyses). Die Suchkriterien fokussierten sich auf mögliche Risikofaktoren für eine avaskuläre Nekrose nach einer suprakondylären Humerusfraktur in der Kindheit. Das Alter, das Geschlecht, die betroffene Seite, die Frakturklassifikation, die Therapie, die Anzahl der K‑Drähte, die Zeit bis zur Operation, Komplikationen sowie die Zeit bis zur Diagnose der avaskulären Nekrose wurden berücksichtigt.
Ergebnisse
Anhand der Suchkriterien wurden 191 Studien ausgewählt, nach Durchsicht der Zusammenfassungen sowie der Volltexte standen 8 Studien zur Verfügung. Von den Patienten waren 18 (60 %) männlich, 11 (36,7 %) weiblich und 1 unbekannt (3,3 %). Das mittlere Alter der Patienten zum Unfallzeitpunkt war 5 Jahre (minimal 2; maximal 10; Standardabweichung, SD: 2,78). Die mittlere Zeit bis zur Diagnosestellung der avaskulären Nekrose betrug 33 Monate (minimal 4; maximal 84; SD: 24,5 Monate). Eine suprakondyläre Humerusfraktur Typ I nach Gartland wiesen 5 Patienten (16,7 %) auf, 3 (10 %) einen Typ II und 22 (73,3 %) einen Typ III. Insgesamt wurden 6 Patienten konservativ mittels einer Gipsschiene und 24 Patienten (80 %) operativ behandelt.
Schlussfolgerung
Die verfügbare Literatur zur avaskulären Nekrose der Trochlea nach pädiatrischen suprakondylären Humerusfrakturen ist begrenzt. Die meisten Patienten waren männlich (60 %) und wiesen eine suprakondyläre Fraktur Typ III nach Gartland (73,3 %) auf. Obwohl eine avaskuläre Nekrose unabhängig vom Frakturtyp auftreten kann, scheint die Schwere der Fraktur ein möglicher Risikofaktor zu sein.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Supracondylar fractures of the humerus are the most common type of elbow fractures in childhood [5, 9]. The peak age at which supracondylar fractures occur is between 5 and 7 years [16].
The majority of these injuries are extension-type fractures accounting for up to 98% of cases. They occur mostly by falling on an outstretched hand with the elbow in full extension especially on the left or non-dominant side [5, 16]. Gartland described a rotatory and translational deformity, with posterior displacement (extension) of the distal fragment occurring most often [5]. He described three types of extension injury based on the degree of displacement—type I, nondisplaced; type II, moderately displaced; and type III, severely displaced injury—and he considered flexion-type injuries separately [5].
Due to the potential risk of severe complications of these fractures, trauma surgeons should address these injuries with caution. Neurovascular injuries are frequent and there may be severe acute complications, which have been reported to occur in up to 12% of displaced fractures [8].
Late complications include secondary deformities due to malunion, usually cubitus varus [13]. However, rarely, other late complications are cubitus valgus, premature epiphyseal arrest, or fishtail deformity [20]. According to the recent data, avascular necrosis of the trochlea after a supracondylar humerus fracture is uncommon and a limited number of cases have been reported in the literature [2, 3, 7, 14, 15, 17]. Hence, the aim of this study was to systematically review the literature in order to evaluate possible predictors for this kind of uncommon late complication of supracondylar distal humerus fractures in childhood.
Methods
In this study, we systematically reviewed randomized clinical trials, observational studies, and case series. We included studies without language restriction. A minimum follow-up of 6 months should be available. We excluded studies evaluating fishtail deformity or trochlea necrosis in other primary injuries (fracture of the medial or lateral condyles). We searched PubMed and Cochrane Library (February 22, 2020) using the search terms:
<predictors> OR <indicators> OR <markers> AND <dissolution> OR <avascular necrosis> OR <nonunion> AND <supracondylar fracture> AND <children> OR <child> OR <Childhood>
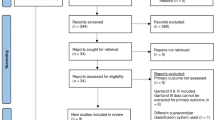
One author (N.O.) conducted the literature research and scanned all references for eligibility. Articles that could not clearly be excluded were retrieved in full-text and read independently by two authors (N.O. and K.W.), who decided independently on eligibility. Disagreements were resolved by discussion. Two authors (N.O. and K.W.) using pre-tested forms extracted study characteristics and results independently. In cases of discrepancies between data in the abstract and the text, we extracted data from the most comprehensive source (Fig. 1). In cases of multiple publications of data from identical patients at different follow-up, we summarized the temporal development and included data from the most recent follow-up only. We did not contact the authors for primary patient data. The available data were organized based on age, sex, affected side, fracture classification, treatment, number of used K‑wires, time to surgery, complications, and time to diagnosis of avascular necrosis.
The risk of bias in observational studies was assessed according to predefined criteria:
-
1.
Cohort clearly defined at baseline
-
2.
Cohort consecutively or randomly sampled
-
3.
Number of drop-outs or loss to follow-up accounted for
-
4.
Outcome blindly assessed
-
5.
Conflicts of interests declared
Statistical analysis
We used descriptive statistics such as frequency distribution to summarize the means and standard deviations. Pearson’s correlation coefficient was used, the level of significance was defined as p <0.05 (SPSS; IBM, Ehningen, Germany).
Results
Overall, 191 articles were found. After reviewing the title and abstract of these articles, 13 out of 191 were selected. After retrieving the full text, eight out of 13 were included in the present study. These studies were mainly retrospective case reports (Fig. 1).
Table 1 summarizes the patients reviewed according to the case reports. Silva et al. reviewed retrospectively the information regarding 143 type II pediatric supracondylar humeral fractures, and avascular necrosis was identified in two patients [18]. Also, Bronfen et al. presented six out of 280 cases with a necrosis of the trochlea after type III Gartland fractures [2]. Etier et al. [3] and Ruiz et al. [17] reported on two patients with an avascular necrosis after a nondisplaced supracondylar humerus fracture. In the study of Etier et al., in four out of five cases it was a Gartland type III fracture. A retrospective report of five cases was presented by Narayanan et al. [14]. Nwakama et al. [15] described two case reports and Kim et al. [10] presented one case report. Glotzbecker et al. reported on eight cases with avascular necrosis of the trochlea after supracondylar humeral fractures [7].
Overall, 30 patients were identified who had avascular necrosis after a supracondylar humerus fracture in childhood. The mean age of injury was 5 years (min. 2; max. 10; SD: 2.8 years); 18 (60.0%) were male, 11 (36.7%) were female, and one was unknown. Six cases (20%) were on the right, nine on the left side (30%), and in 15 out of 30 (50%) the side was unknown. Five patients (16.7%) had a Gartland type I, three (10.0%) a type II, and 22 (73.3%) a type III fracture of the distal humerus (Table 2). Six patients (20.0%) were treated nonoperatively, whereas 24 patients (80.0%) underwent operative treatment with closed reduction and K‑wire fixation (23) or an open reduction and K‑wire fixation (1; Table 3). An accompanying nerve lesion was reported in 23.3% of cases (n = 7): The radial nerve (n = 4), the median nerve (n = 2), and the ulnar nerve (n = 1) were affected. The surgical procedure was known in 13 out of 30 cases. In 33.3% of patients (n = 10), two lateral K‑wires were used, while in only 10% patients (n = 3) was an additional medial K‑wire needed. The time to surgery was available in 24 cases. It was less than 7 days in 22 cases (91.7%) and more than 7 days in two cases (8.3%).
The avascular necrosis of the trochlea was diagnosed at a mean time of 33 months (min. 4; max. 84; SD: 24.5 months). Fracture type correlated with the accompanying complications, especially nerve lesions (r = 0.436; p = 0.042), and with the therapy selected (r = 0.666; p = 0.00). The other variables showed no significant correlations. The majority of the patients with avascular necrosis were males and had displaced supracondylar humerus fracture, Gartland type III. The severity of the fracture with the amount of dislocation may be a risk factor for avascular necrosis.
Nevertheless, we were not able to identify any significant predictors for avascular necrosis after supracondylar humerus fractures in childhood.
Discussion
We reviewed the literature on 30 patients with avascular necrosis after a supracondylar humerus fracture in childhood. The supracondylar humerus fracture is a common injury of the pediatric elbow, whereas avascular necrosis is a rarely reported complication [2, 3]. Hence, it could be a misdiagnosed long-term complication. The patients had a mean age at injury of 5 years, 4–10 years is a typical age for these fractures [5, 16]. The majority were displaced fractures, Gartland type III [5, 6]. However, avascular necrosis was also identified after non-displaced fractures of the distal humerus in some cases [17]. The lack of blood supply seems to represent a potential risk for avascular necrosis. A recent study investigated the vascular anatomy of the distal humerus and demonstrated that the distal metaphysis of the humerus is consistently supplied by a single, terminal, nutrient artery, terminating approximately 3–4 cm proximal to the olecranon fossa. Areas distal to this point are considered a watershed area. In particular, the medial column is supplied by anterior and posterior segmental perforating vessels, without anastomosis between the medial and lateral perforating vessels [11, 20].
Beaty and Kasser classified two types of trochlea necrosis depending on the vascular supply. Type A necrosis is the result of an injury to the posterior vessels supplying the lateral aspect of the trochlea, whereas type B necrosis results from an insult to both the medial and lateral vascular supplies of the trochlea [1]. These investigations underline the severity of the fracture as a possible risk factor. According to our data, it remains unclear whether the initial trauma and/or the surgical procedure caused a lack of blood supply to the capitulum. Hence, only one out of 24 patients had an open reduction and K‑wire osteosynthesis. Moreover, only three patients were treated with an additional medial K‑wire. Thus, relevant injury to the blood vessels that supply the trochlea, as possibly seen in extended surgical approaches, seems improbable [4, 19]. Our results may indicate that the severity of the fracture and not the surgical procedure is a potential risk for avascular necrosis.
However, avascular necrosis was also observed in non-displaced supracondylar fractures. Yang et al. studied the vascularization of 19 fetuses to establish a hypothesis for avascular necrosis [21]. There was a longitudinal channel of vessels in the epiphysis between the capitulum and the trochlea, a centripetal vascularity in the area of muscle attachment, and a vascular arch in the olecranon fossa that also nourished the epiphyseal cartilage of the distal humerus. The vessels are susceptible to all types of fractures, including a non-displaced fracture, because of the longitudinal nature. Nevertheless, a severely displaced fracture will lead to a pronounced interruption of the vascularization.
The mean delay of 33 months between the fracture and the development of changes is consistent with avascular necrosis. The trochlea ossifies at the age of 7–8 years [12], and the defect of the lateral trochlea cannot be seen because the medial part is still cartilaginous. Thus, avascular necrosis would not be evident on radiographs of younger children. The patients present mainly with restricted range of motion, pain, or crepitus. Radiography and magnetic resonance imaging should be performed whenever avascular necrosis is suspected. Depending on the severity of the presenting symptoms, a conservative procedure can be attempted. However, it is essential to keep the patient under observation. In some cases, a complete regeneration of small defects with minimal joint involvement can be observed.
In the case of persistent symptoms, an operative management should be discussed. Depending on the individual pathology, the operative treatment ranges from arthroscopic or open debridement with capsulotomy, to surgical arrest of the remaining medial or lateral epiphysis and to osteotomy for persistent deformity. However, recurrent symptoms were not uncommon and long-term outcomes are missing [7]. Consequently, the parents, the child, and the surgeon should have a frank discussion on the possible operative and conservative procedures available. With the knowledge that the natural course of necrosis cannot be influenced, it appears to be more important to identify these patients early in the course of the disease. Our own experience supports the results of this study as seen in the following clinical case.
Case report
A 4-year-old girl suffered a fall on her right elbow while she was playing on the street, causing a Gartland type III supracondylar fracture (Fig. 2). The physical examination indicated pain, severe swelling, and functional impairment. No sensorimotor deficit was present. The patient was immediately treated operatively by closed reduction and internal fixation with crossed K‑wires (Fig. 3). The K‑wires were removed 9 weeks postoperatively after fracture union had occurred. Three years later, the patient presented with restricted range of motion in the right elbow. She had 30 ° of extension and 110 ° of flexion. Radiography showed demineralization and decreased size of the ossification center of the trochlea and a transient osteochondrosis was suspected (Fig. 4). We discussed the option of a conservative procedure and arthroscopic debridement. After careful consideration, we decided to attempt a conservative procedure. The patient was kept under observation. In the case of persistent symptoms, operative management should be discussed.
Limitations
The present systematic review has some limitations. First, it included only 30 patients with avascular necrosis. Some vital information was missing in the literature. Thus, the summary provided in the systematic review of the literature is only as reliable as the methods used to estimate the effect in each of the primary studies.
Practical conclusion
-
The body of literature does not give adequate evidence on the topic of avascular necrosis.
-
Avascular necrosis after supracondylar humerus fractures is an uncommon complication but should not be underestimated.
-
The majority of patients with avascular necrosis were male and had a displaced supracondylar humerus fracture, Gartland type III.
-
The severity of the fracture with the amount of dislocation is a possible risk factor for avascular necrosis.
-
The mean time to diagnosis was 33 months and the mean age at the time of injury was 5 years.
-
Avascular necrosis was reported mainly after an operative treatment but some cases were observed after non-displaced fractures.
-
The treatment of avascular necrosis should be based on the symptoms and is an individual decision.
-
The goal is to identify these patients early in the course of disease in order to lessen the severity of deformity.
References
Beaty J, Kasser J (2006) The elbow: physeal fractures, apophyseal injuries of the distal humerus, osteonecrosis of the trochlea, and T‑condylar fractures. In: Rockwood C, Wilkins KE (eds) Rockwood and Wilkins’ fractures in children, 6th edn. Lippincott Williams & Wilkins, Philadelphia, pp 592–610
Bronfen CE, Geffard B, Mallet JF (2007) Dissolution of the trochlea after supracondylar fracture of the humerus in childhood: an analysis of six cases. J Pediatr Orthop 27:547–550
Etier BE Jr, Doyle JS, Gilbert SR (2015) Avascular necrosis of trochlea after supracondylar humerus fractures in children. Am J Orthop (Belle Mead NJ) 44(10):E390–3
Fowles JV, Kassab MT (1974) Displaced supracondylar fractures of the elbow in children. A report on the fixation of extension and flexion fractures by two lateral percutaneous pins. J Bone Joint Surg Br 56:490–500
Gartland JJ (1963) Supracondylar fractures of the humerus. Med Trial Tech Q 10:37–46
Gartland JJ (1959) Management of supracondylar fractures of the humerus in children. Surg Gynecol Obstet 109:145–154
Glotzbecker MP, Bae DS, Links AC et al (2013) Fishtail deformity of the distal humerus: a report of 15 cases. J Pediatr Orthop 33:592–597
Gosens T, Bongers KJ (2003) Neurovascular complications and functional outcome in displaced supracondylar fractures of the humerus in children. Injury 34:267–273. https://doi.org/10.1016/S0020-1383(02)00312-1
Graham HA (1967) Supracondylar fractures of the elbow in children. Clin Orthop Relat Res 54:93–102
Kim HT, Song MB, Conjares JN et al (2002) Trochlear deformity occurring after distal humeral fractures: magnetic resonance imaging and its natural progression. J Pediatr Orthop 22:188–193
Kimball JP, Glowczewskie F, Wright TW (2007) Intraosseous blood supply to the distal humerus. J Hand Surg Am 32:642–646
McCarthy S, Ogden JA (1982) Radiology of postnatal skeletal development. V. distal humerus. Skelet Radiol 7:239–249
Morrissy RT, Wilkins KE (1984) Deformity following distal humeral fracture in childhood. J Bone Joint Surg Am 66:557–562
Narayanan S, Grottkau BE, Nimkin K (1992) Fishtail deformity—a delayed complication of distal humeral fractures in children. Orthopedics 15(8):959–963
Nwakama AC, Peterson HA, Shaughnessy WJ (2000) Fishtail deformity following fracture of the distal humerus in children: historical review, case presentations, discussion of etiology, and thoughts on treatment. J Pediatr Orthop B 9:309–318
Pouliquen JC (1987) Fractures du coude de l’enfant. Rev Chir Orthop Reparatrice Appar Mot 73:418–489
Ruiz Picazo D, Ramírez Villaescusa J, Delgado García AB (2015) Dissolution of the capitellum after nondisplaced supracondylar fracture of the humerus in a child: a case report of transient osteochondrosis. J Pediatr Orthop B 24(3):219–222. https://doi.org/10.1097/BPB.0000000000000161
Silva M, Wong TC, Bernthal NM (2011) Outcomes of reduction more than 7 days after injury in supracondylar humeral fractures in children. J Pediatr Orthop 31(7):751–756. https://doi.org/10.1097/BPO.0b013e31822f16e5
Toniolo RM, Wilkins EK (1996) Avascular necrosis of the trochlea. In: Rockwood CA Jr, Wilkins KE, Beaty HB (eds) Fractures in children, 4th edn. vol 3. Lippincott Williams & Wilkins, Philadelphia, pp 821–830
Wegmann K, Burkhart KJ, Koslowsky TC, Koebke J, Neiss WF, Müller LP (2014) Arterial supply of the distal humerus. Surg Radiol Anat 36(7):705–711. https://doi.org/10.1007/s00276-013-1240-z
Yang Z, Wang Y, Gilula LA, Yamaguchi K (1998) Microcirculation of the distal humeral epiphyseal cartilage: implications for post-traumatic growth deformities. J Hand Surg Am 23:165–172
Funding
Open Access funding enabled and organized by Projekt DEAL.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
N. Ott, M. Hackl, T. Leschinger, K. Wegmann and L.P. Müller declare that they have no competing interests.
For this article no studies with human participants or animals were performed by any of the authors. All studies performed were in accordance with the ethical standards indicated in each case.
Rights and permissions
Open Access. This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Ott, N., Hackl, M., Leschinger, T. et al. Predictors of avascular necrosis of the trochlea after pediatric supracondylar humerus fractures. Obere Extremität 15, 301–306 (2020). https://doi.org/10.1007/s11678-020-00606-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11678-020-00606-9