Abstract
Background
Prosthetic replacement of the proximal humerus with reverse shoulder arthroplasty (RSA) is an established means of treatment. Due to its unique biomechanical characteristics, RSA can restore shoulder function to a satisfying level in the case of cuff tear arthropathy, arthritis, and fractures. However, complications are frequent in RSA, one of the more common being implant instability with dislocation. The present study investigated the influence of glenosphere diameter and metaglene lateralization using a unique test setup.
Methods
Seven fresh-frozen cadaveric specimens of the shoulder were thawed and dissected. The subscapularis muscle, the infraspinatus, and the three heads of the deltoid muscle were fixed to a pulley system. After implanting an RSA in different configurations (38/42 mm glenosphere with lateralization of +0 mm, +5 mm, or +10 mm), the implants were dislocated using selective muscle pull. The frequency of dislocations depending on the prosthesis configuration was documented.
Results
The larger glenosphere diameter of 42 mm showed less dislocations than the diameter of 38 mm (39 vs. 46). Lateralization of +0, +5 mm, and +10 mm showed 26, 29, and 30, dislocations, respectively. Dislocation via pull on the infraspinatus muscle was most frequent. None of the results reached statistical significance.
Conclusion
The current investigation used a novel technique for investigating the effect of lateralization and glenosphere diameter on RSA instability. Despite indicating tendencies, the present test setup could not prove the hypothesis that a larger glenosphere diameter and increased lateralization add to stability. The lack of statistical significance could be attributable to the low specimen number. The clinical significance of lateralization and glenosphere diameter should be further assessed in future biomechanical investigations.
Zusammenfassung
Hintergrund
Der Ersatz des proximalen Humerus mittels inverser Prothesen ist eine etablierte Therapiemaßnahme. Aufgrund der besonderen biomechanischen Eigenschaften kann eine suffiziente Schulterfunktion auch bei irreparablen Manschettendefekten, Arthrose und komplexen Frakturen wiederhergestellt werden. Eine häufigere Komplikation ist die Instabilität. Die vorliegende Studie untersuchte den Einfluss des Glenosphärendurchmessers und der Lateralisierung des Glenoids auf die Stabilität einer inversen Schulterprothese.
Methodik
Bei 7 unfixierten menschlichen Leichenpräparaten wurde eine biomechanische Untersuchung durchgeführt. An den Präparaten wurden die Muskeln der Rotatorenmanschette sowie die drei Köpfe des M. deltoideus freigelegt und über ein Rollensystem angeschlungen. Nach Implantation einer inversen Schulterprothese in unterschiedlichen Konfigurationen (Glenosphäre 38/42 mm; Lateralisierung der Glenosphäre um +0 mm, +5 mm und +10 mm) erfolgte die gezielte Luxation über selektiven Muskelzug. Untersucht wurde die Häufigkeit der Luxationen in Abhängigkeit von den Prothesenkonfigurationen.
Ergebnisse
Ein größerer Glenosphärendurchmesser von 42 mm resultierte in einer geringeren Luxationsrate gegenüber einem Durchmesser von 38 mm. Bei einer Lateralisierung von +0, +5 mm und +10 mm zeigten sich 26, 29 bzw. 30 Luxationen. Am häufigsten führte der Zug am M. infraspinatus zur Luxation. Keines der Ergebnisse war statistisch signifikant.
Schlussfolgerung
Die vorliegende Studie nutzte eine innovative Methodik zur Untersuchung der Effekte der Lateralisierung und des Glenosphärendurchmessers auf die Instabilität einer inversen Schulterprothese. Trotz erkennbarer Tendenzen konnte die Annahme, dass ein größerer Glenosphärendurchmesser und mehr Lateralisation zur Stabilität beitragen, nicht bestätigt werden. Denkbar ist, dass eine höhere Anzahl an Leichenpräparaten zu einer stärkeren Abgrenzung der einzelnen Gruppen führen würde.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Reverse shoulder arthroplasty (RSA) has gained a prominent role in the treatment of unreconstructable fractures of the proximal humerus as well as of other pathologies such as cuff tear arthropathy and failure of anatomical shoulder prostheses [4, 5, 9, 10, 29]. By shifting the center of rotation (COR), the Grammont design and the more recent reverse shoulder prosthesis designs achieve an improvement of the abductor lever arm of the deltoid muscle, therefore compensating for deficiencies of the cuff or loss of the tuberosities [5, 9, 10]. Although reverse prostheses are used in growing numbers, they are accompanied by high rates of complications of up to 60% [2, 3, 11]. Besides infections, periprosthetic fractures, and loosening, a common cause of failure of reverse prostheses is instability [5, 13, 15]. Soft tissue balancing is known to play a major role in shoulder replacement, especially for stability. Furthermore, bone loss is common in many indications for reverse prostheses and is a known contributor to instability [13, 15]. As RSA does not replicate normal joint anatomy, implant selection is a key factor in gaining joint stability by tensioning the deltoid muscle and the remaining soft tissues. Glenosphere diameter has been investigated for its role in range of motion in reverse joint reconstruction [1, 14, 28] and for decreasing scapular notching [26], but also for its role in joint tensioning and stability [20, 24]. A further tool to influence soft tissue tensioning is lateralization of the glenosphere and the COR compared to the implant designed by Grammont, which has been investigated in recent biomechanical papers such as that of Ferle et al. in 2019 [12]. The present study aimed to investigate the influence of glenosphere diameter and metaglene lateralization on stability using a dynamic test setup with preloading of the rotator cuff and the deltoid. It was hypothesized that a larger glenosphere diameter and increased lateralization will add to stability, while excess lateralization might lead to dislocation of the prosthesis due to over-tensioning of the deltoid.
Materials and methods
Specimens
All procedures involving human cadavers were performed in accordance with the ethical standards of the institutional research committee (Ethical Committee of the Medical Faculty of the University of Cologne, VT 19-1540).
For the present study, seven fresh-frozen cadaveric shoulder specimens were available. Four left and three right shoulder specimens were used. Mean age of body donors at time of death was 71 years (minimum 52, maximum 87; standard deviation [SD] 12.8). Prior to testing, the specimens were thawed at room temperature. Using fluoroscopy, the specimens were checked for preexisting pathologies including fracture sequelae with bony deformity or implants already present. The specimens included the shoulder joint with scapula, clavicle, and the proximal half of the humerus, as well as the intact soft tissue envelope.
Reverse shoulder arthroplasties and implant selection
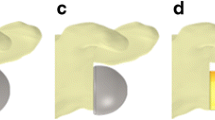
All RSA were implanted using the deltopectoral approach. For each cadaveric shoulder the DELTA XTEND Cementless Modular Humeral Implant RSA (DePuy Synthes Companies, Warsaw, IN, USA) was used. To test the specimens in the scenario of cuff deficiency, the supraspinatus tendon was severed while the subscapularis (SSC) and infraspinatus (ISP) were preserved. Osteotomy of the humeral head was performed in 10° retroversion using the systemʼs cutting guide. Thereafter, the canal of the humerus was reamed and a standard-length humeral stem was press-fit implanted. The metaglene was prepared and the baseplate was fixed in position with two 3.5-mm non-locking screws. The glenosphere (38 mm or 42 mm according to the group) was then placed with a neutral offset. To allow for incremental lateralization, polymethyl methacrylate (PMMA) spacers of incremental thickness were formed and were prepared to be placed under the baseplate. Spacers were used in sizes of +5 mm and +10 mm (Fig. 1). To increase lateralization the baseplates were augmented without a spacer (+0 mm), with a spacer of +5 mm, and with a spacer of +10 mm. To additionally be able to investigate the influence of glenosphere diameter, the testing was first performed with the 38-mm glenosphere implanted and then performed again with the 42-mm glenosphere. In all cases an inlay +3 mm of either 38 mm or 42 mm diameter was used. The origins of the SSC, ISP, and deltoid muscles were sectioned proximally and each origin was augmented with finger traps and a #2 Fiberwire (Arthrex Inc., Naples, FL, USA). The Fiberwire sutures were passed close to their bony insertions in a medial direction, beyond the body of the scapula, to optimally replicate their force vector (Fig. 2). Medially to the scapula, the sutures were connected to force transmitters, allowing incremental loading of the respective muscles (Fig. 3).
Exemplification of the test setup. The body of the scapula (BS) is potted (P) and fixed to the test bench. Here seen from ventral, the ventral (DV) and acromial (DA) sections of the deltoid are augmented. The augmentation technique with finger traps and Fiberwire sutures (Arthrex Inc., Naples, FL, USA) is shown. Infraspinatus and dorsal deltoid are at the back, subscapularis has been resected for visibility (CL clavicle, H humerus)
The trajectory of the finger traps and the Fiberwire sutures (Arthrex Inc., Naples, FL, USA) is shown. The sutures are passed along their force vector through a mounting plate, which serves as a pulley. Beyond the plate the respective weights were attached. DA acromial section of the deltoid, DV ventral section of the deltoid, SSC subscapularis
Biomechanical testing protocol
For the experimental setup, the body of the scapula was fixed rigidly in a custom-made holding device and the tendons were attached along the force vector of the attached muscles using a guided pulley. The three segments of the deltoid (pars clavicularis, acromialis, and spinalis) were addressed separately. To test the stability of the available implant combinations, the aim was to use loading of the muscles to bring the cadaveric specimens into positions that would lead to dislocation. Therefore, the augmented muscles were loaded in a load-to-failure manner. As a first step of each testing cycle, the augmented muscles (SSC, ISP, deltoid pars clavicularis and spinalis) were preloaded with 10 N and the deltoid (pars acromialis) with 40 N. The preload brought the specimens into a floating abduction position of 40° and allowed re-establishment of pre-tensioning of the soft tissues.
By adding incremental load onto one muscle (either the SSC, ISP, ventral or dorsal deltoid) in steps of 500 g, increasing rotation (external rotation when loading ISP/dorsal deltoid or internal rotation when loading SSC/ventral deltoid) was achieved. While doing so, the aforementioned preloads where left on the other muscles. The loads were increased to the point that the implant dislocated or to the maximum of 5 kg (49.05 N).
This testing protocol was performed with a 38-mm glenosphere (group I) with lateralizations of +0 mm, +5 mm, and +10 mm. The protocol was then repeated identically with a 42-mm glenosphere (group II) and incremental lateralization. The muscle load necessary to cause dislocation was noted as the peak dislocation force and the number of dislocations were counted in the respective groups.
Statistical analysis
Descriptive statistics were computed to summarize the means and standard deviations. Normal distribution of the data was tested using the Kolmogorov-Smirnov test. An analysis of variance (ANOVA) test was used to detect statistically significant differences. The level of significance was set at a p-value of <0.05. Statistical analysis was performed using SSPS statistics software (version 25.0.0.0; IBM, Armonk, NY, USA).
Results
In this study, peak dislocation force was used as a measure of stability. The presented mean values are based on the applied weight (kg) for dislocation. The descriptive statistics are given in Table 1. The table gives the number of dislocations, while the descriptive values refer to the respective force that was applied on the muscles. The boxplots give an overview of these data and display the SD graphically (Fig. 4). The ANOVA test showed p-values >0.05 for all dependencies; no statistically significant differences were found.
Discussion
The present study did not reveal any significant findings regarding the influence of glenosphere diameter and lateralization on the stability of reverse total shoulder arthroplasty in an in vitro setting. In comparison to a glenosphere diameter of 38 mm, a glenosphere diameter of 42 mm showed less dislocation events in the respective load groups; nevertheless, the level of significance was not reached. Lateralization did not further improve stability using the given test setup.
While the configuration of an RSA can be altered by many design parameters, the present study focused on the two variables of glenosphere lateralization and diameter, as the authors believe these to be highly influential for soft tissue tensioning, joint load, and thereby stability. Previous basic science studies could show that an increase in glenosphere diameter influences joint load [20, 21]. Langohr et al. investigated the effects of glenosphere diameter on reverse shoulder prosthetic implants and found an increase in joint load as well as in deltoid force with larger diameters. Joint load does not directly equate to joint stability. The latter study group did not investigate joint stability with their model. Indeed, only few cadaver studies have elaborated specifically on the effects of glenosphere diameter and lateralization on joint stability [6, 12, 17, 24]. In 2012, Henninger et al. published their work on the effects of progressive lateralization on dislocation forces of RSA in a cadaveric shoulder simulator [17]. They dislocated the implants by applying a direct pull using a line. The group reported that increasing forces were necessary to dislocate the implant with increasing lateralization. However, in their dataset, they found statistical significance in only a subset of the cases and groups. Clouthier et al. performed a similar investigation with a custom shoulder simulator, using saw bones as specimens [6]. The authors investigated the influence of glenosphere eccentricity as well as of socket depth on stability and found that the necessary force for joint dislocation increased with both. The group also applied the dislocating force directly via a line connected to the humerus at the implant–bone interface. Clouthier et al. did not investigate the influence of lateralization or glenosphere diameter on stability. Pastor et al. used an elaborate robot-based test setup to investigate the influence of rotator cuff condition and glenosphere diameter on RSA stability [24]. They found a 32% increase in dislocation force for the 42-mm glenosphere compared to the 38-mm glenosphere. Also, there were higher forces necessary for dislocation of the implants when the rotator cuff was left intact. The authors did not investigate the effects of lateralization on RSA stability. The group of Ferle, Pastor, and Smith recently published a biomechanical investigation on the topic using a similar cadaver setup with a robotic shoulder simulator, including the effect of humeral component characteristics and glenosphere lateralization on stability after RSA [12]. They found significantly higher construct stability when the glenosphere was lateralized by 6 or 9 mm. Ferle’s robotic shoulder simulator allowed the shoulders to be positioned and held and the dislocating force to be applied. Their setup obviously allows precise measurement and a high level of repeatability. While the latter authors used direct pull of the rotator cuff and the deltoid muscle in their native force vector for implant dislocation, the current study aimed for an alternative experimental dislocation maneuver.
It is reasonable to assume muscle force to be a driving factor in real-life implant dislocations, e.g., in external rotation and abduction or maximum internal rotation. To the best of the authorsʼ knowledge, this is the first biomechanical study to apply such a technique in a cadaveric in vitro model of shoulder specimens. The study indicates glenosphere size to be a stabilizing component modification in RSA but does not give statistical evidence. In addition, it did not confirm the data of previous biomechanical studies where lateralization contributed directly to implant stability. This might be due to methodological flaws. Potentially, a higher number of specimens might have allowed a clearer distinction between the groups, as only seven specimens were available for investigation. Specimen count is typically low, as availability and costs play a deciding role in this regard. Also, peak dislocation force was used as a measure of joint stability. This has been used in previous work [6], but does not allow the whole spectrum of instability and micromotion to be taken into account. Therefore, aspects of subtler implant instability were potentially missed in the investigation. It would be of interest to repeat the setup with a motion-tracking device to analyze implant stability in more detail. Moreover, one has to bear in mind that the mechanical test setup with static muscle loading in cadaveric specimens does not fully match the biological muscle response. Further, it must be noted that when using the subscapularis and infraspinatus muscles as the dislocating drive, the respective results can only be drawn into comparisons for shoulders where the respective muscles are intact. This is often not the case for shoulders with an indication for RSA. In the present setup, preloading of the anterior and posterior cuff was necessary to balance the joint in the floating abduction position that resulted from the deltoid preload. However, when applying the dislocating forces, the forces were a multitude higher than the preload of the cuff and probably did not affect the results negatively. Also, no differences in the results were found between the two groups where the deltoid or the cuff was loaded until dislocation.
While biomechanical studies on the subject have shown measurable effects on joint load and improvement of joint stability [7, 12, 23], a systematic review of clinical studies by Helmkamp et al. did not find significant differences in terms of dislocations and instability between lateralized and standard COR implants [16]. Summarizing the results, the authors reported improved external rotation and reduced scapular notching in the group of implants with lateralized COR. However, no significant differences regarding complications or outcome scores were found, except for a higher metaglene loosening rate in the lateralized group. These results of Helmkamp’s group basically confirmed the reported data from the systematic review of Lawrence et al. from 2016 [22], who also reported better external rotation with less scapular notching. Notably, both reviews found a higher rate of metaglene loosening in the lateralized group [16, 22], which is in accordance with basic research from Yang et al. and Denard et al. [8, 30]. Yang et al. investigated the effect of glenosphere diameter and eccentricity on shear forces acting on the metaglene baseplate in RSA [30]. Their 2D finite element study convincingly showed an increase of stress at the base of the metaglene, at the inferior screw, with increasing metaglene eccentricity and offset. In a 3D finite element setup, Denard et al. investigated stress and displacement at the metaglene in RSA, depending on glenosphere diameter and lateralization [8]. Notably, their setup differentiated between bony lateralization and lateralization directly via the metaglene component. They found a significant increase of stress and displacement at the metaglene with increasing diameter of the glenosphere and with lateralization, while the stress increased more strongly with bony lateralization than with prosthetic lateralization. These studies support the clinical findings of the reports by Lawrence et al. [22] and Helmkamp et al. [16], as increased mechanical stress is a reasonable explanation for loosening and, ultimately, failure. Zumstein’s group reported a significantly higher rate of aseptic loosening of the metaglene component in a lateralized implant compared to a medialized COR group [31]. Besides metaglene stress, it has been found that lateralization also results in increased tension in the deltoid muscle or increased force needed to abduct the arm [18,19,20]. Hettrich et al. concluded from their finite element study that lateralization led to improvements in range of motion but also resulted in increased force needed to abduct the arm [18], which itself may lead to pronounced deltoid fatigue and acromial stress fractures [25, 27].
Conclusion
Although instability is a common issue in RSA and glenosphere diameter and lateralization represent tools to counter this, optimizing joint load or stability comes with tradeoffs. Therefore, further biomechanical insight and close monitoring of clinical results are necessary.
References
Berhouet J, Garaud P, Favard L (2014) Evaluation of the role of glenosphere design and humeral component retroversion in avoiding scapular notching during reverse shoulder arthroplasty. J Shoulder Elbow Surg 23:151–158
Bois AJ, Knight P, Alhojailan K et al (2020) Clinical outcomes and complications of reverse shoulder arthroplasty used for failed prior shoulder surgery: a systematic review and meta-analysis. JSES Int 4:156–168
Botros M, Curry EJ, Yin J et al (2019) Reverse shoulder arthroplasty has higher perioperative implant complications and transfusion rates than total shoulder arthroplasty. JSES Open Access 3:108–112
Castagna A, Delcogliano M, De Caro F et al (2013) Conversion of shoulder arthroplasty to reverse implants: clinical and radiological results using a modular system. Int Orthop 37:1297–1305
Churchill JL, Garrigues GE (2016) Current controversies in reverse total shoulder arthroplasty. JBJS Rev. https://doi.org/10.2106/JBJS.RVW.15.00070
Clouthier AL, Hetzler MA, Fedorak G et al (2013) Factors affecting the stability of reverse shoulder arthroplasty: a biomechanical study. J Shoulder Elbow Surg 22:439–444
Costantini O, Choi DS, Kontaxis A et al (2015) The effects of progressive lateralization of the joint center of rotation of reverse total shoulder implants. J Shoulder Elbow Surg 24:1120–1128
Denard PJ, Lederman E, Parsons BO et al (2017) Finite element analysis of glenoid-sided lateralization in reverse shoulder arthroplasty. J Orthop Res 35:1548–1555
Drake GN, O’connor DP, Edwards TB (2010) Indications for reverse total shoulder arthroplasty in rotator cuff disease. Clin Orthop Relat Res 468:1526–1533
Ek ET, Neukom L, Catanzaro S et al (2013) Reverse total shoulder arthroplasty for massive irreparable rotator cuff tears in patients younger than 65 years old: results after five to fifteen years. J Shoulder Elbow Surg 22:1199–1208
Erickson BJ, Shishani Y, Jones S et al (2020) Outpatient vs. inpatient reverse total shoulder arthroplasty: outcomes and complications. J Shoulder Elbow Surg. https://doi.org/10.1016/j.jse.2019.10.023
Ferle M, Pastor MF, Hagenah J et al (2019) Effect of the humeral neck-shaft angle and glenosphere lateralization on stability of reverse shoulder arthroplasty: a cadaveric study. J Shoulder Elbow Surg 28:966–973
Gauci MO, Cavalier M, Gonzalez JF et al (2020) Revision of failed shoulder arthroplasty: epidemiology, etiology, and surgical options. J Shoulder Elbow Surg 29:541–549
Gutierrez S, Comiskey CA, Luo ZP et al (2008) Range of impingement-free abduction and adduction deficit after reverse shoulder arthroplasty. Hierarchy of surgical and implant-design-related factors. J Bone Joint Surg Am 90:2606–2615
Hasler A, Fornaciari P, Jungwirth-Weinberger A et al (2019) Reverse shoulder arthroplasty in the treatment of glenohumeral instability. J Shoulder Elbow Surg 28:1587–1594
Helmkamp JK, Bullock GS, Amilo NR et al (2018) The clinical and radiographic impact of center of rotation lateralization in reverse shoulder arthroplasty: a systematic review. J Shoulder Elbow Surg 27:2099–2107
Henninger HB, Barg A, Anderson AE et al (2012) Effect of lateral offset center of rotation in reverse total shoulder arthroplasty: a biomechanical study. J Shoulder Elbow Surg 21:1128–1135
Hettrich CM, Permeswaran VN, Goetz JE et al (2015) Mechanical tradeoffs associated with glenosphere lateralization in reverse shoulder arthroplasty. J Shoulder Elbow Surg 24:1774–1781
Hoenecke HR Jr., Flores-Hernandez C, D’lima DD (2014) Reverse total shoulder arthroplasty component center of rotation affects muscle function. J Shoulder Elbow Surg 23:1128–1135
Langohr GD, Giles JW, Athwal GS et al (2015) The effect of glenosphere diameter in reverse shoulder arthroplasty on muscle force, joint load, and range of motion. J Shoulder Elbow Surg 24:972–979
Langohr GD, Willing R, Medley JB et al (2016) Contact mechanics of reverse total shoulder arthroplasty during abduction: the effect of neck-shaft angle, humeral cup depth, and glenosphere diameter. J Shoulder Elbow Surg 25:589–597
Lawrence C, Williams GR, Namdari S (2016) Influence of Glenosphere design on outcomes and complications of reverse arthroplasty: a systematic review. Clin Orthop Surg 8:288–297
Middleton C, Uri O, Phillips S et al (2014) A reverse shoulder arthroplasty with increased offset for the treatment of cuff-deficient shoulders with glenohumeral arthritis. Bone Joint J 96-b:936–942
Pastor MF, Kraemer M, Wellmann M et al (2016) Anterior stability of the reverse shoulder arthroplasty depending on implant configuration and rotator cuff condition. Arch Orthop Trauma Surg 136:1513–1519
Pegreffi F, Pellegrini A, Paladini P et al (2017) Deltoid muscle activity in patients with reverse shoulder prosthesis at 2‑year follow-up. Musculoskelet Surg 101:129–135
Roche C, Flurin PH, Wright T et al (2009) An evaluation of the relationships between reverse shoulder design parameters and range of motion, impingement, and stability. J Shoulder Elbow Surg 18:734–741
Walch G, Mottier F, Wall B et al (2009) Acromial insufficiency in reverse shoulder arthroplasties. J Shoulder Elbow Surg 18:495–502
Werner BS, Chaoui J, Walch G (2018) Glenosphere design affects range of movement and risk of friction-type scapular impingement in reverse shoulder arthroplasty. Bone Joint J 100-B:1182–1186
Wieser K, Borbas P, Ek ET et al (2015) Conversion of stemmed hemi- or total to reverse total shoulder arthroplasty: advantages of a modular stem design. Clin Orthop Relat Res 473:651–660
Yang CC, Lu CL, Wu CH et al (2013) Stress analysis of glenoid component in design of reverse shoulder prosthesis using finite element method. J Shoulder Elbow Surg 22:932–939
Zumstein MA, Pinedo M, Old J et al (2011) Problems, complications, reoperations, and revisions in reverse total shoulder arthroplasty: a systematic review. J Shoulder Elbow Surg 20:146–157
Funding
For the present study funds were received from the German Society for Shoulder and Elbow Surgery (DVSE).
Funding
Open Access funding provided by Projekt DEAL.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
K. Wegmann, A. Alikhah, T. Leschinger, A. Harbrecht, L. P. Müller, M. Hackl, and N. Ott declare that they have no competing interests.
All procedures performed in studies involving human participants or on human tissue were in accordance with the ethical standards of the institutional research committee (Ethical Committee of the Medical Faculty of the University of Cologne, VT 19-1540).
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Wegmann, K., Alikhah, A., Leschinger, T. et al. Influence of glenosphere diameter and lateralization on instability of reverse shoulder arthroplasty. Obere Extremität 15, 199–206 (2020). https://doi.org/10.1007/s11678-020-00593-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11678-020-00593-x